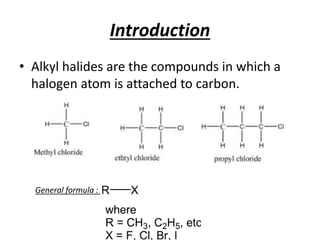
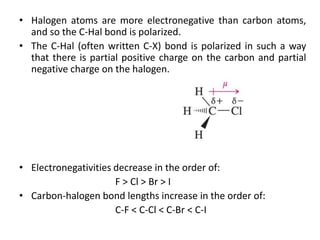
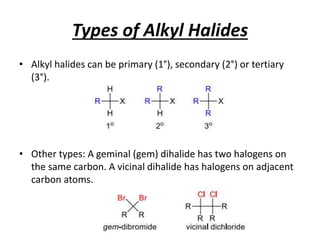
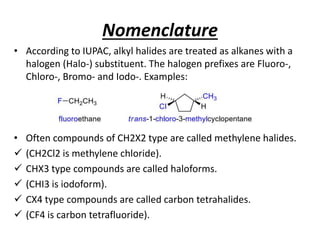
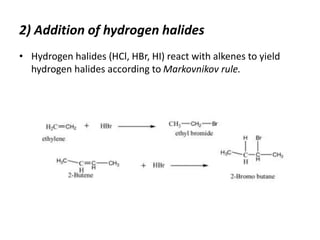
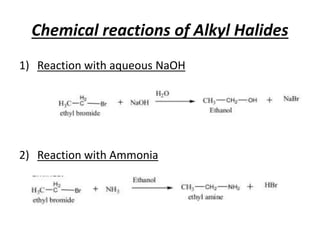
Alkyl halides are organic compounds where a halogen atom is bonded to a carbon. They are polarized with partial positive charge on carbon and partial negative on the halogen. Alkyl halides can be primary, secondary or tertiary depending on the carbon they are bonded to. They are prepared through halogenation of alkanes, addition of hydrogen halides to alkenes/alkynes, or reaction of alcohols with halogenating agents. As the halogen is a good leaving group, alkyl halides undergo substitution and elimination reactions. They find use as anesthetics, refrigerants, pesticides, and in other applications.