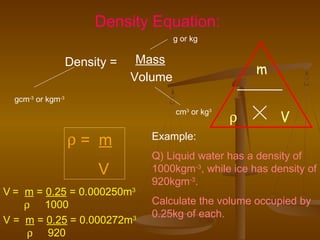
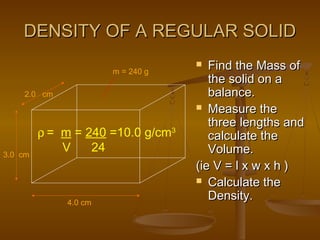
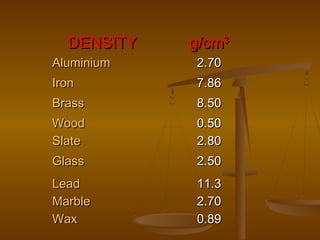
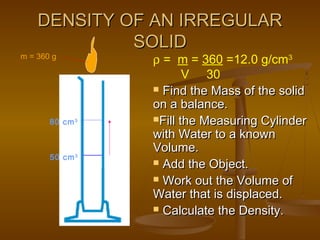
Here are the steps Jake can take to find the density of the book using the tools he has:
1. Use the balance to measure the mass of the book. Let's say it's 1,000 grams.
2. Use the ruler to measure the dimensions of the book - its length, width, and height.
3. Calculate the volume of the book by multiplying its length by its width by its height. For example, if the book is 20 cm long, 10 cm wide, and 2 cm thick, its volume would be 20 cm x 10 cm x 2 cm = 400 cm3.
4. Use the density formula: Density = Mass / Volume
5. Plug in the values: