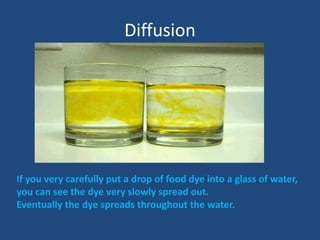
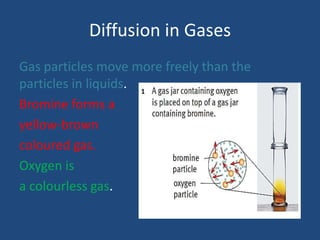
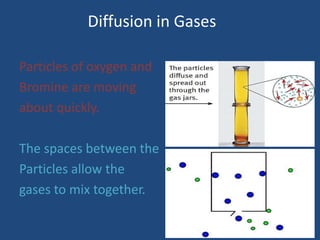
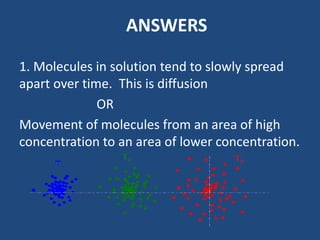
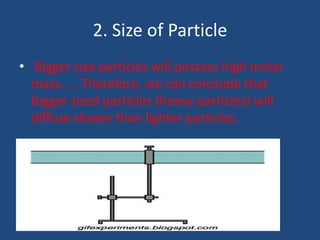
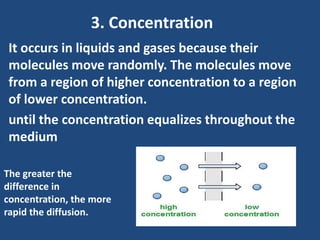
The document explains diffusion, defining it as the movement of molecules from an area of high concentration to one of lower concentration. It discusses diffusion in liquids and gases, highlighting that gas particles move more freely and quickly than those in liquids, affected by factors such as temperature, particle size, and concentration. The summary concludes that the rate of diffusion is influenced by these factors, with lighter particles diffusing faster than heavier ones.