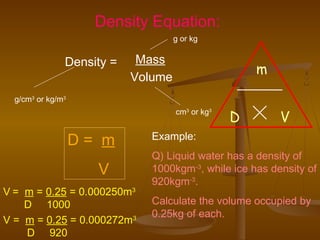
1. Density is defined as mass per unit volume and can be used to compare how much mass is contained within a certain volume of different materials.
2. The density of a material can be calculated by measuring its mass and volume, with density equaling mass divided by volume.
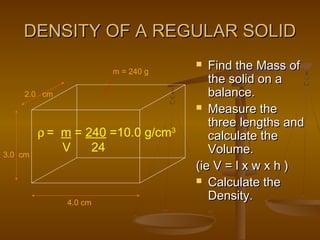
3. There are different methods to determine the density of regular solids, irregular solids, liquids, and gases depending on whether their volume needs to be measured directly or determined through displacement.