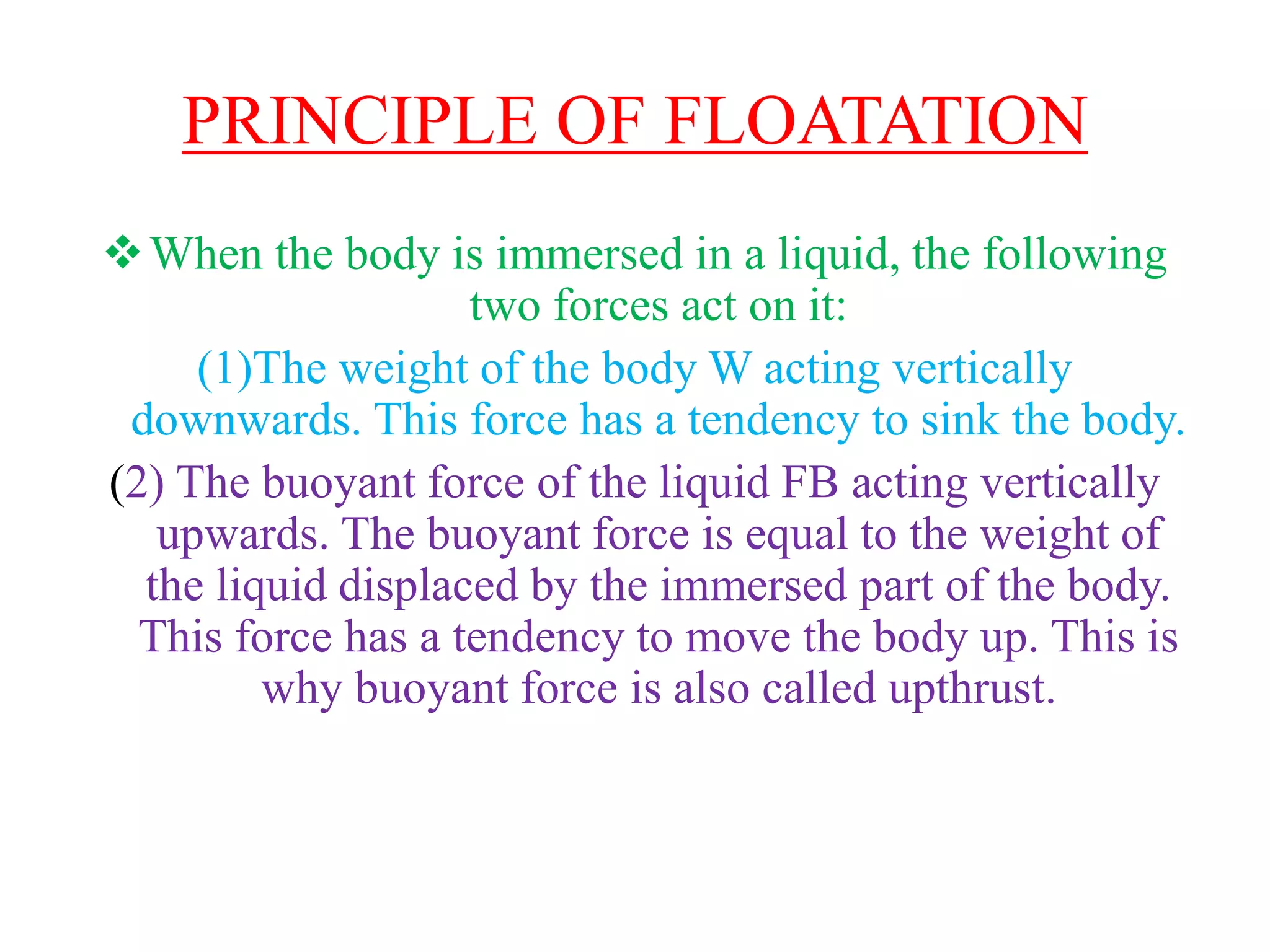
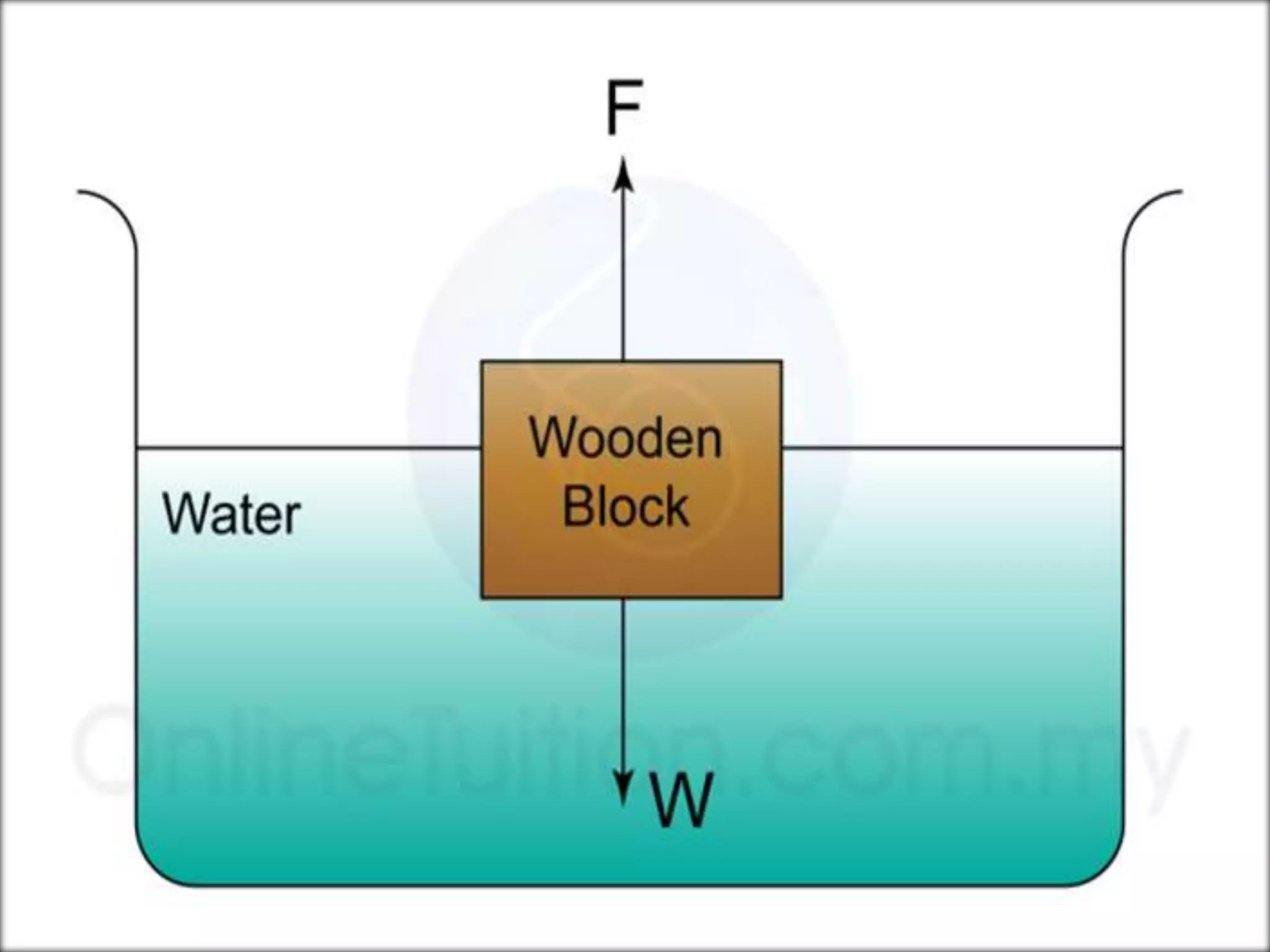
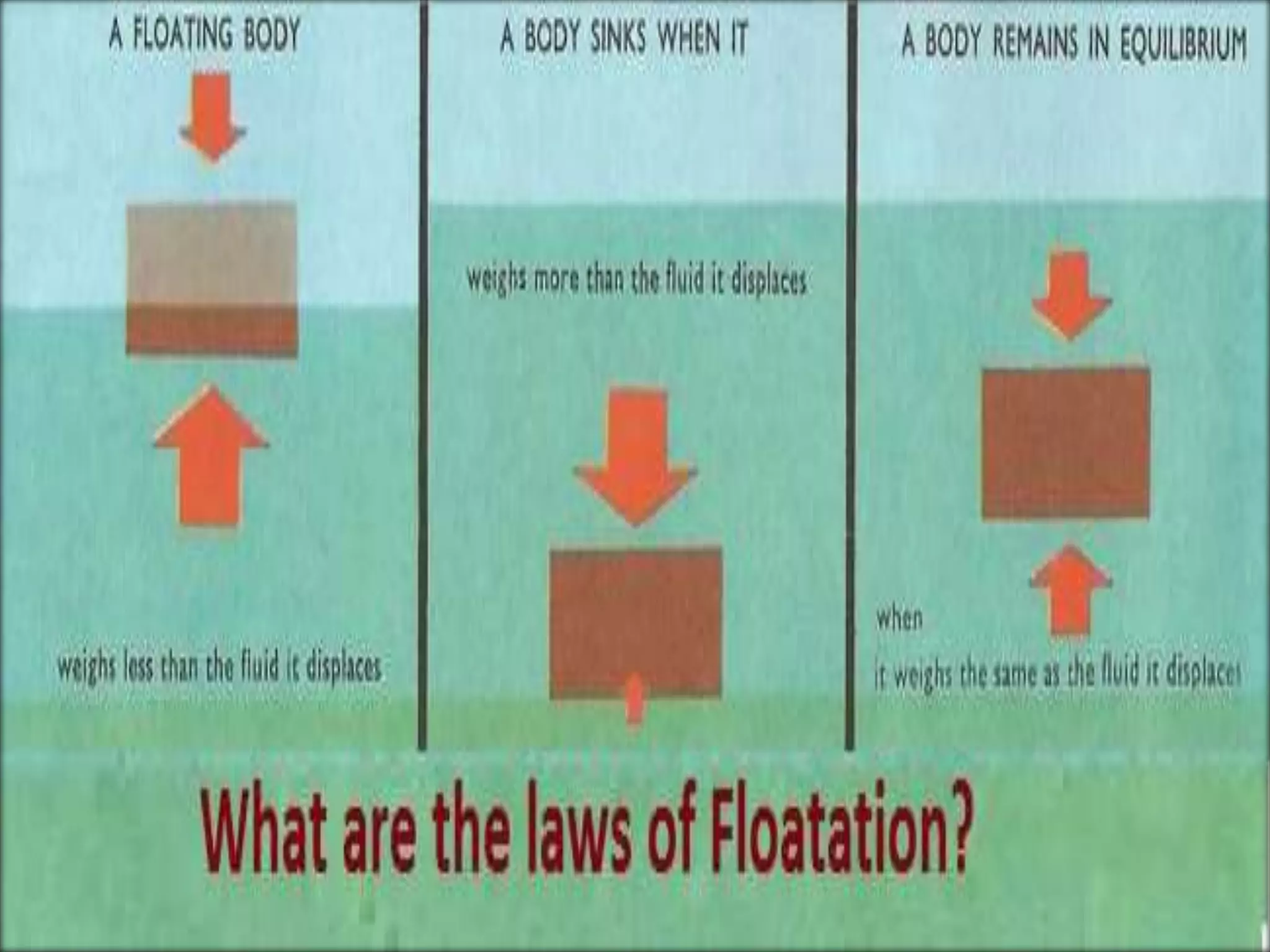
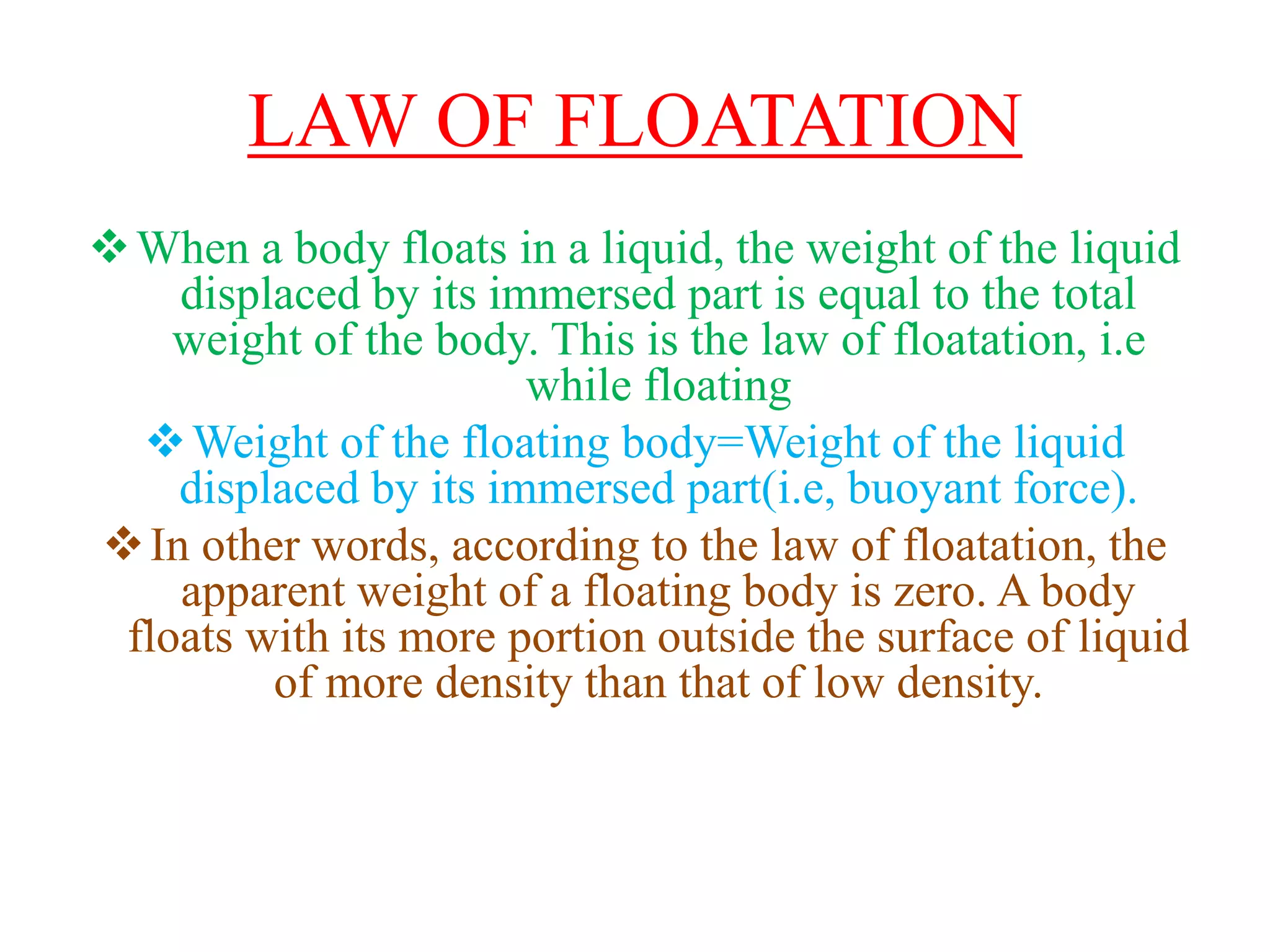
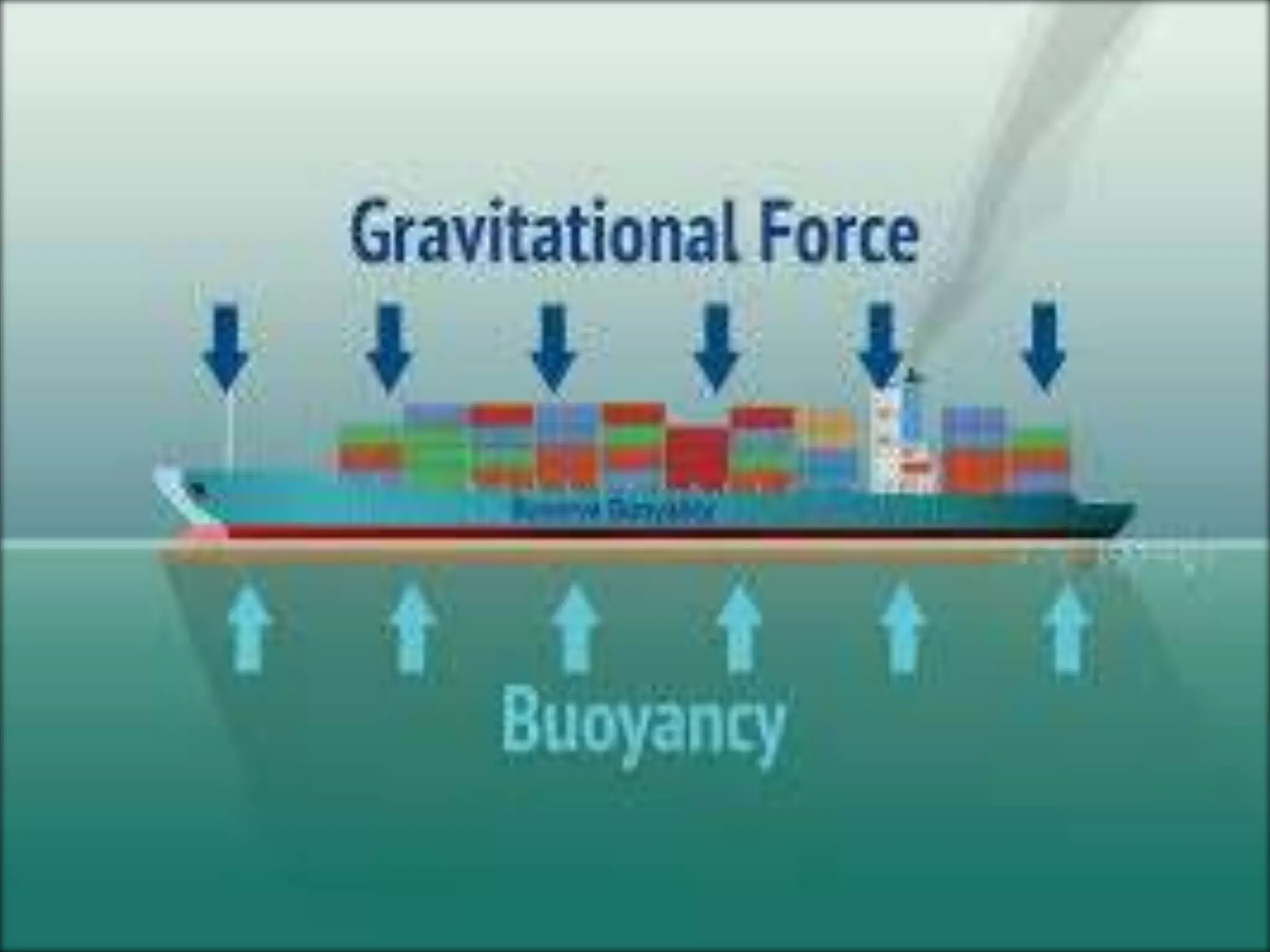
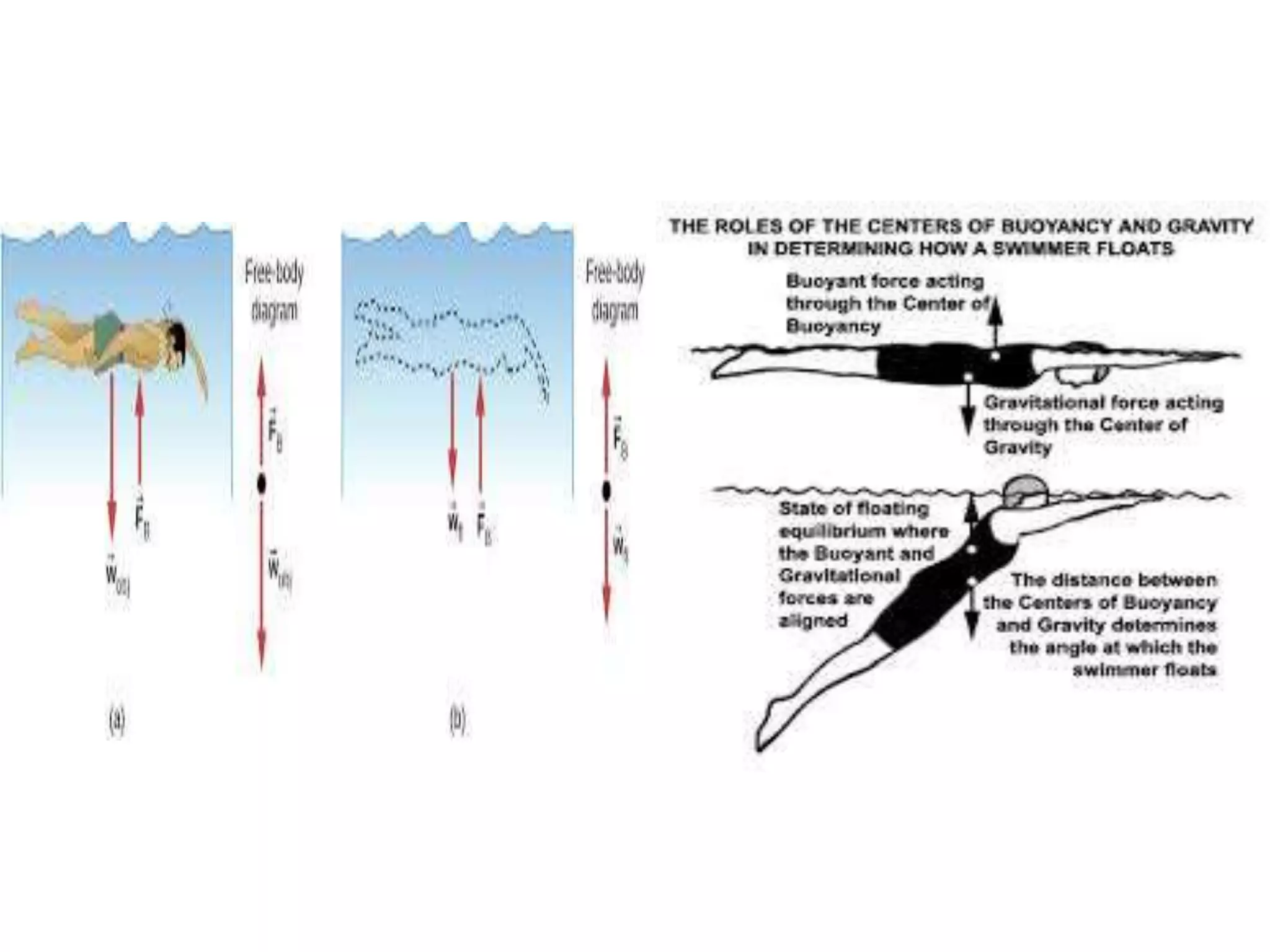
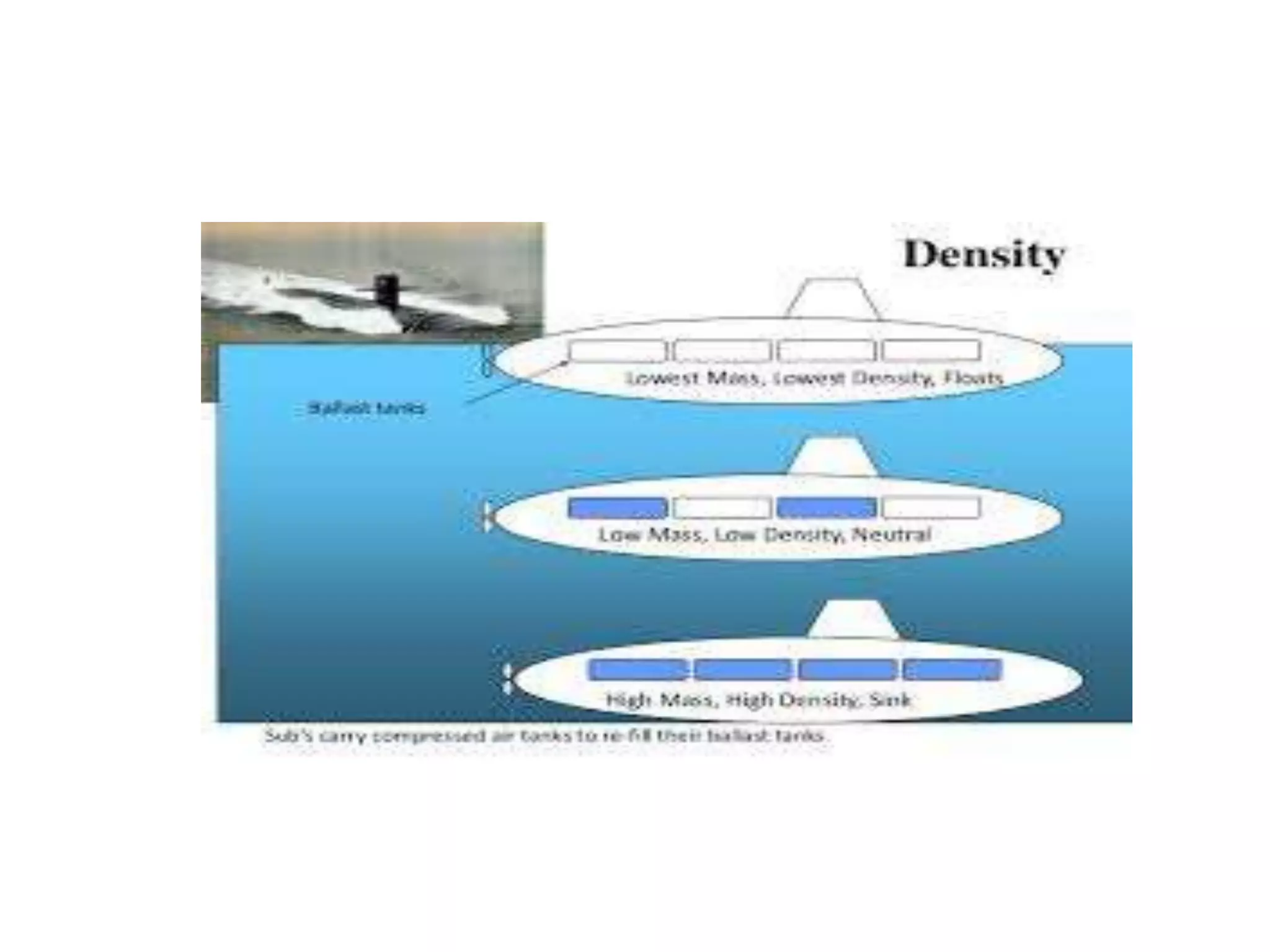
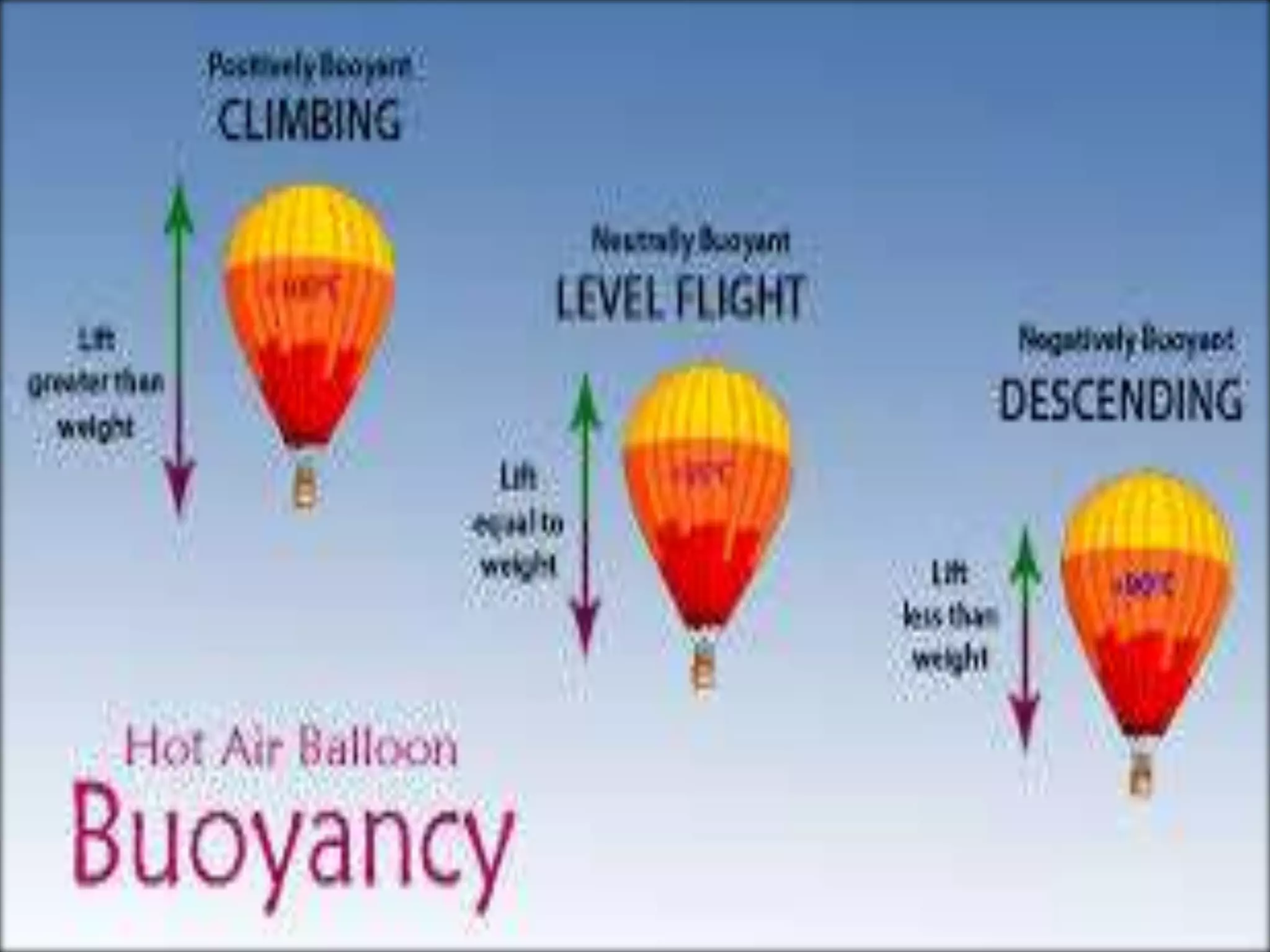
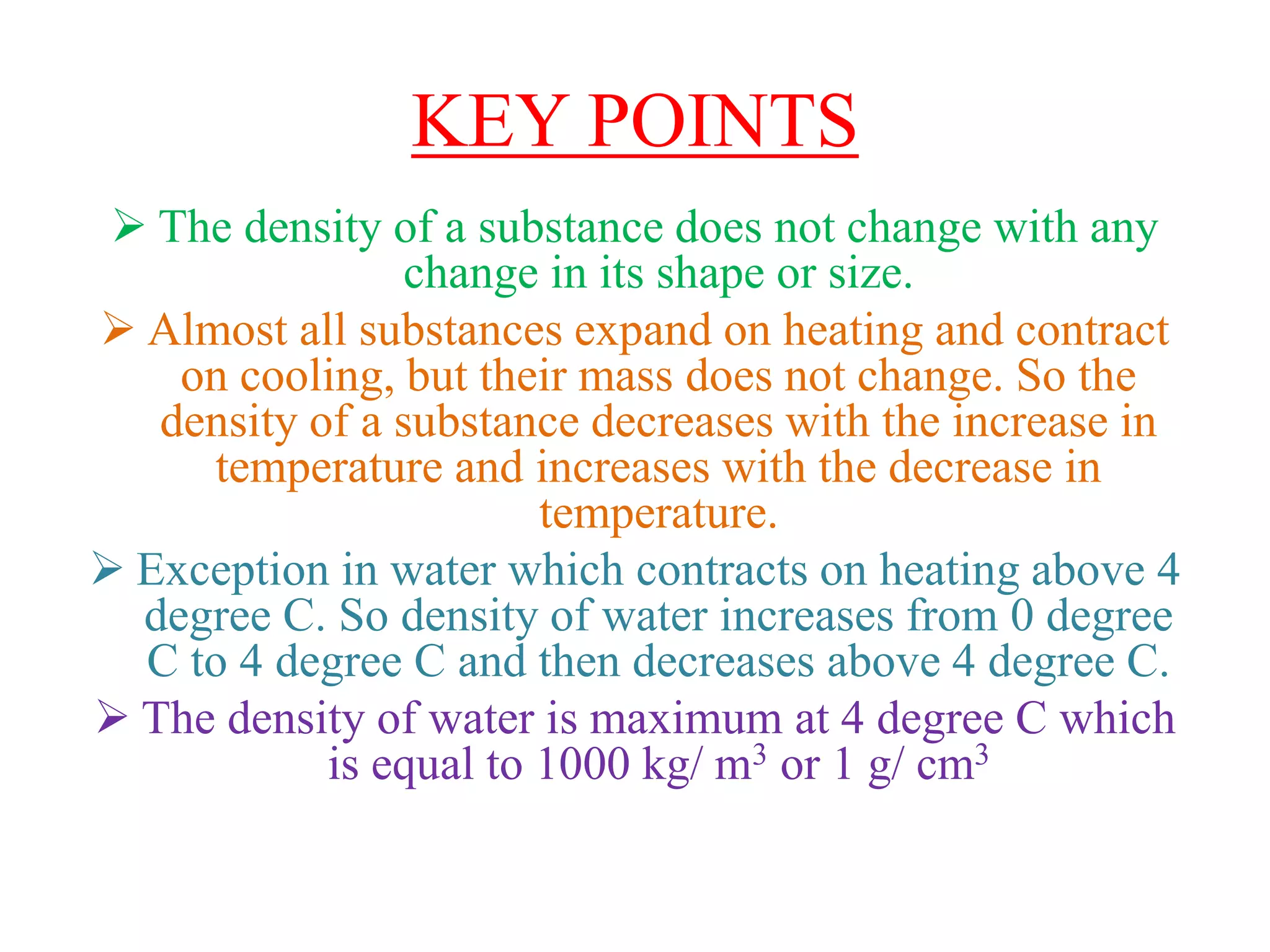
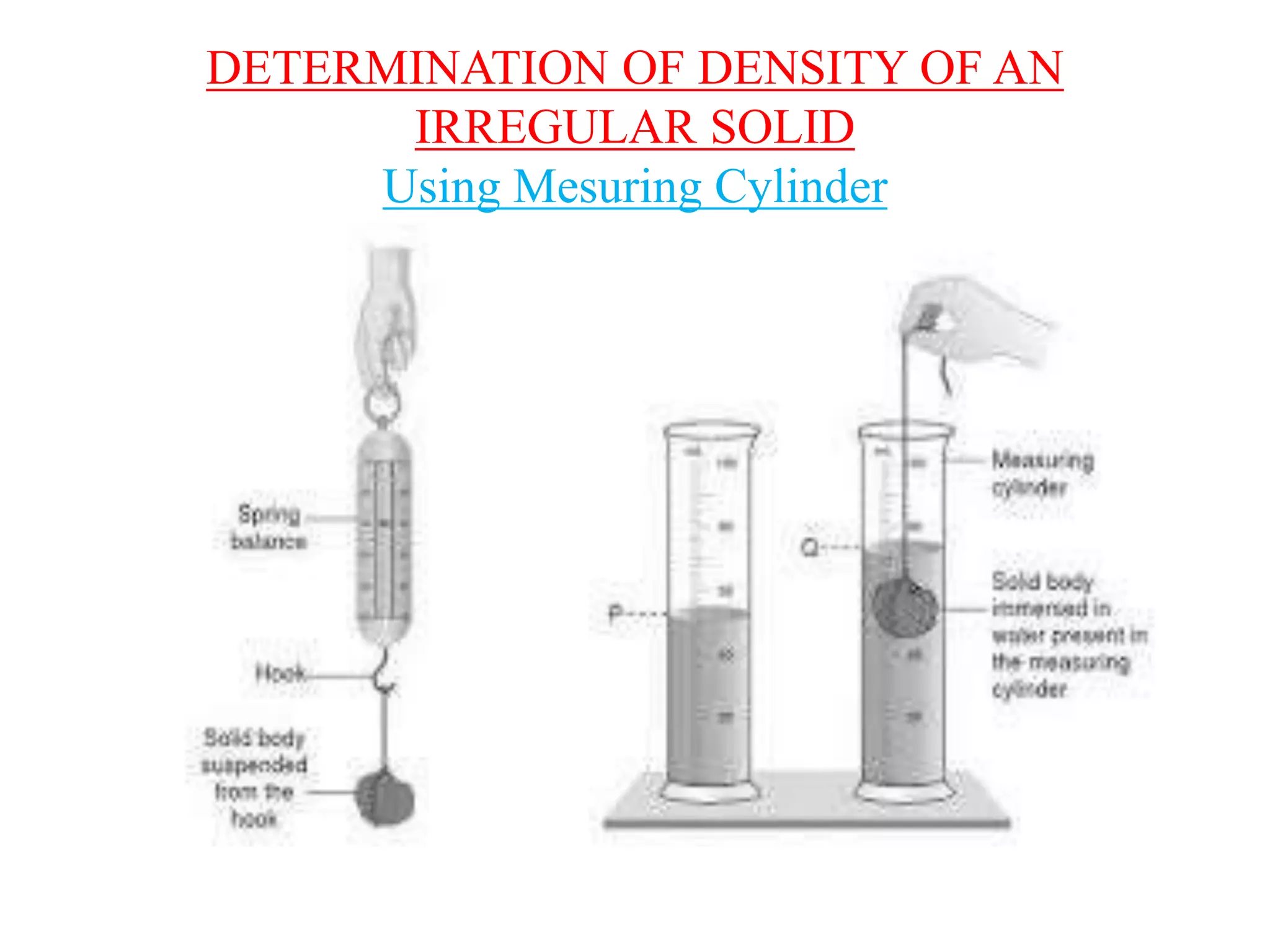
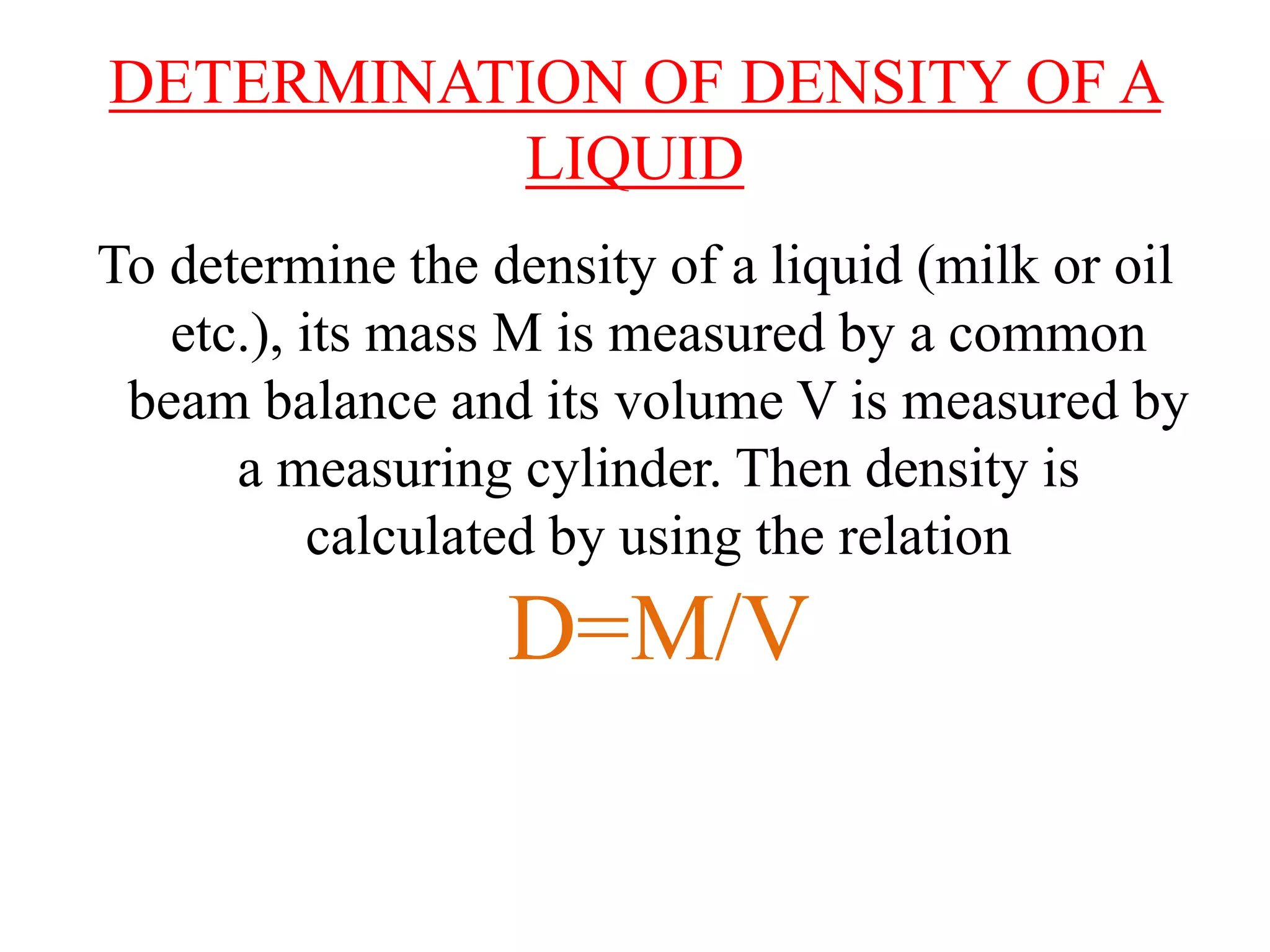
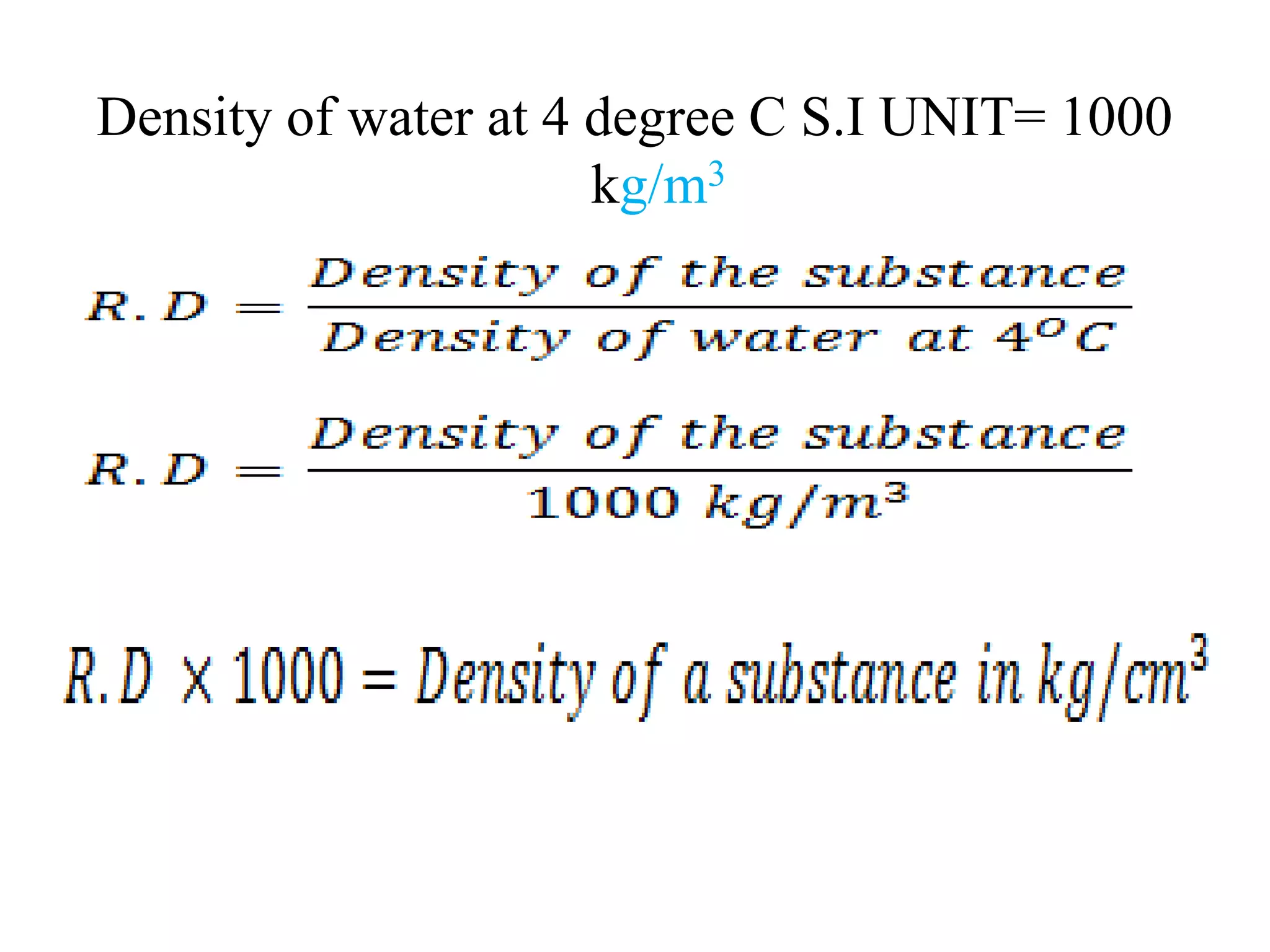
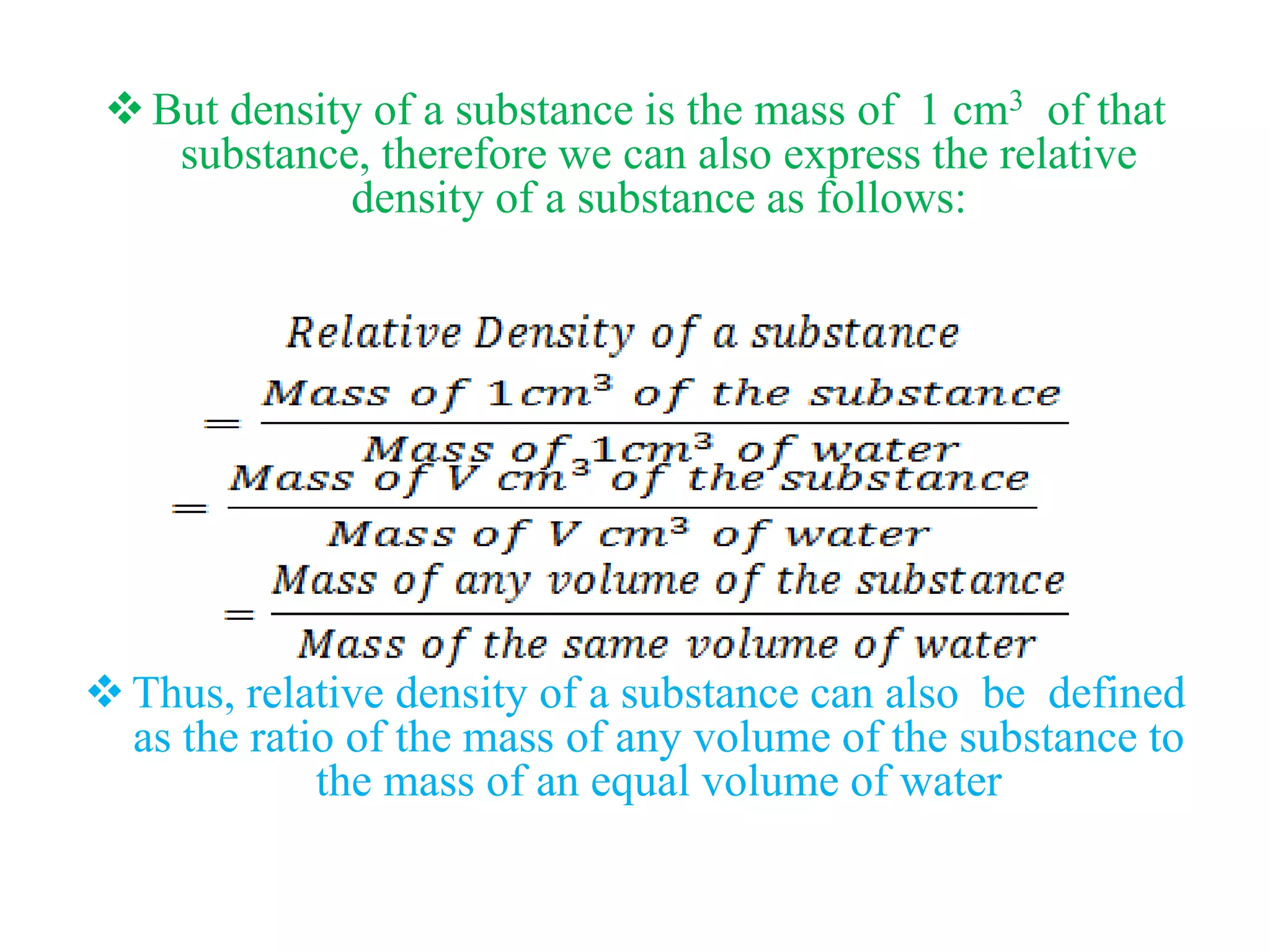
This document provides information about a physics class covering physical quantity and measurement, density, and floatation. It defines density as the mass per unit volume and provides conversions between kg/m3 and g/cm3. It describes methods to measure the density of irregular solids and liquids using a measuring cylinder or Eureka can. The principles of floatation and applications such as ships, swimming, and submarines are also summarized. Numerical problems are included for practice calculations.















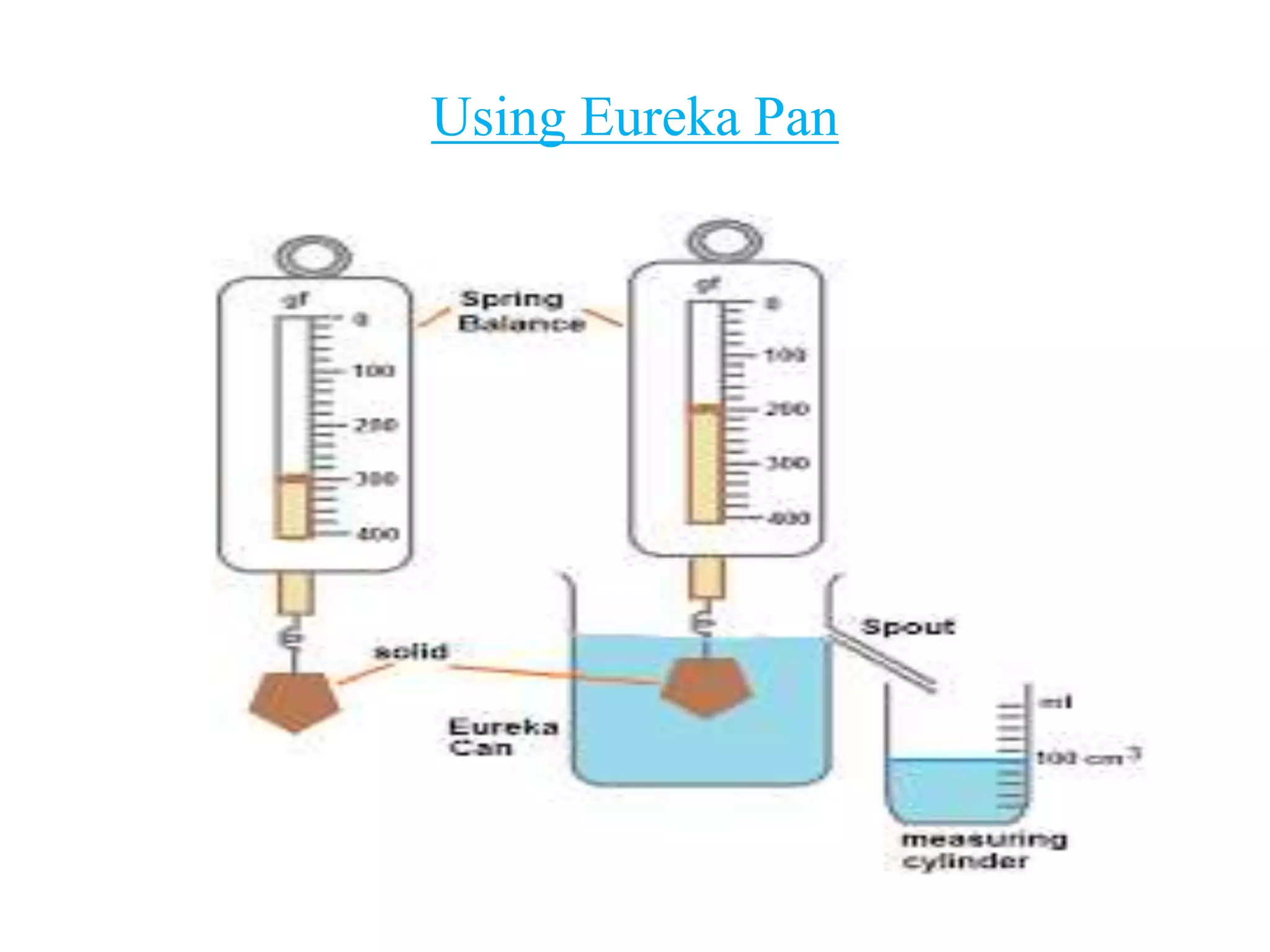
![ Pour water into the can until it starts overflowing through the
spout. When the water has stopped dripping, remove the
measuring cylinder. Empty it, dry it and again place it under
the spout.
Now tie the irregular solid by a thread. Immerse the solid
gently into the water contained in the Eureka can. The solid
displaces water equal to its own volume which overflows
through the spout and gets collected in measuring cylinder.
When water stops dripping out through the spout, note the
volume of water collected in the measuring cylinder [100
cm3]
Dry the solid. Measure the mass M of the given solid with a
bam balance/ spring balance.
Let the mass be 300 g.
Volume of the solid (V) = Volume of water collected in the
measuring cylinder= 100 cm3
Density = M/V = 300/100 = 3 g/cm3](https://image.slidesharecdn.com/class-8physicalquantitymeasurement2-201107073556/75/CLASS-8-PHYSICAL-QUANTITY-MEASUREMENT-19-2048.jpg)
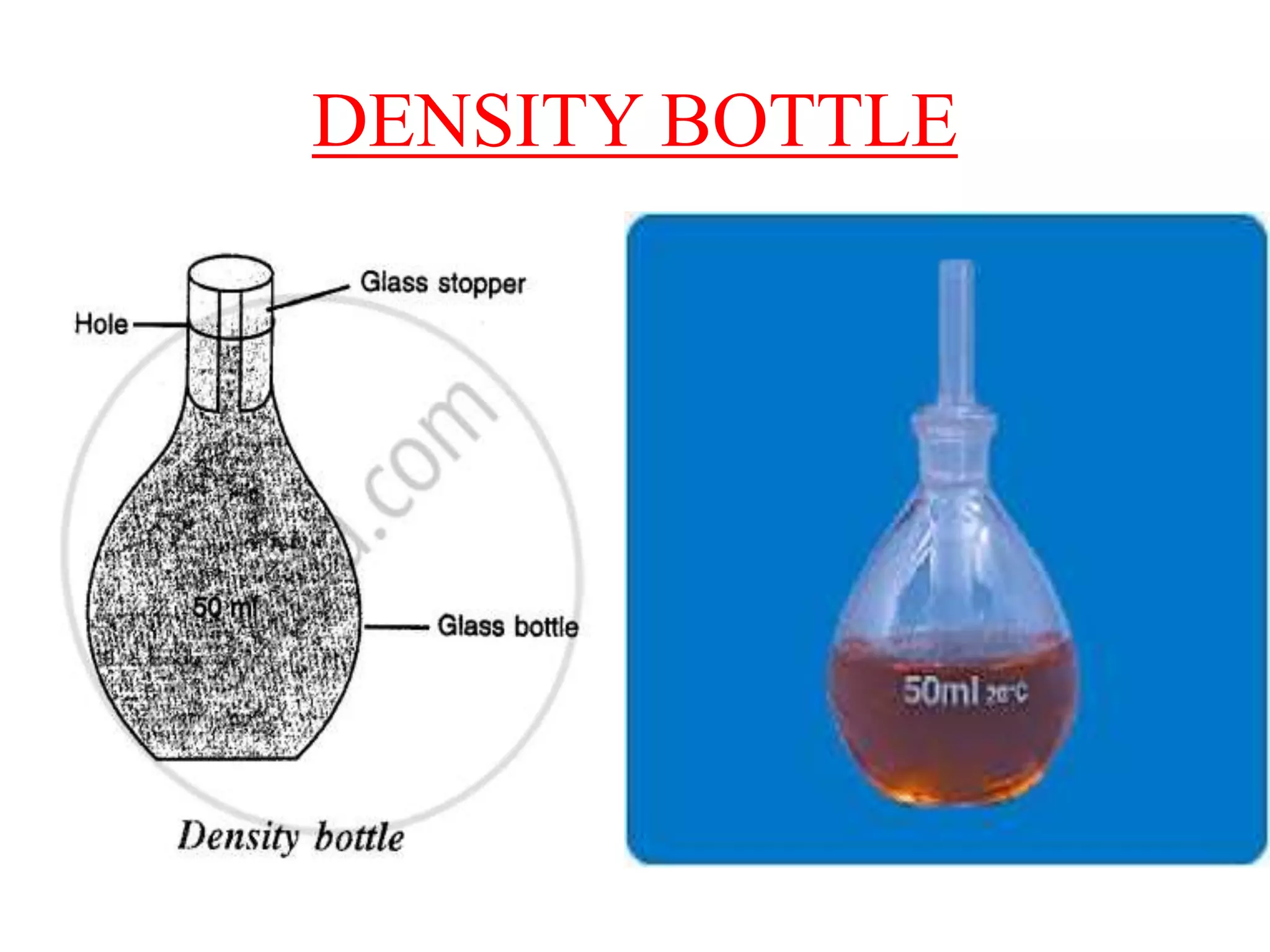


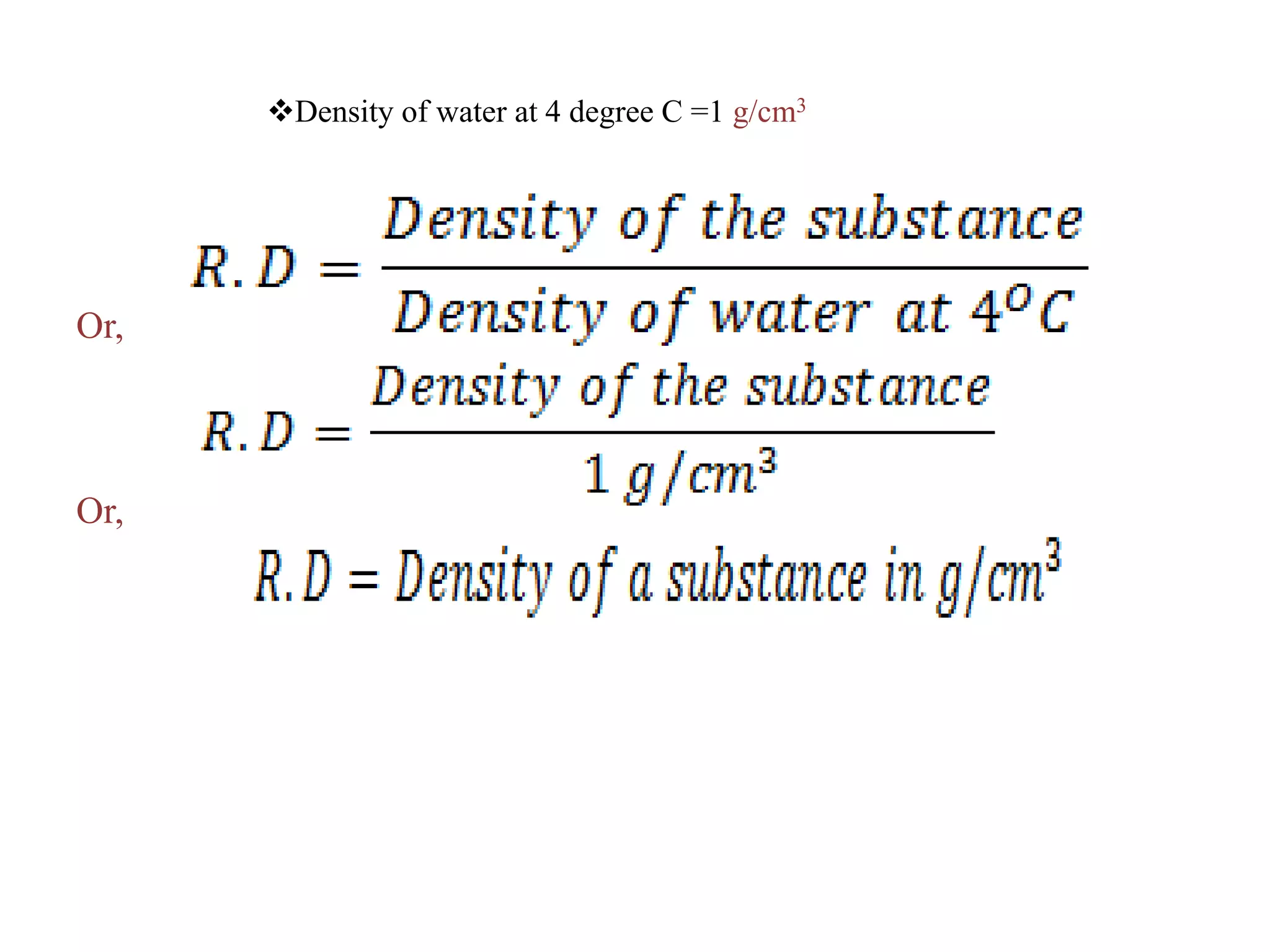
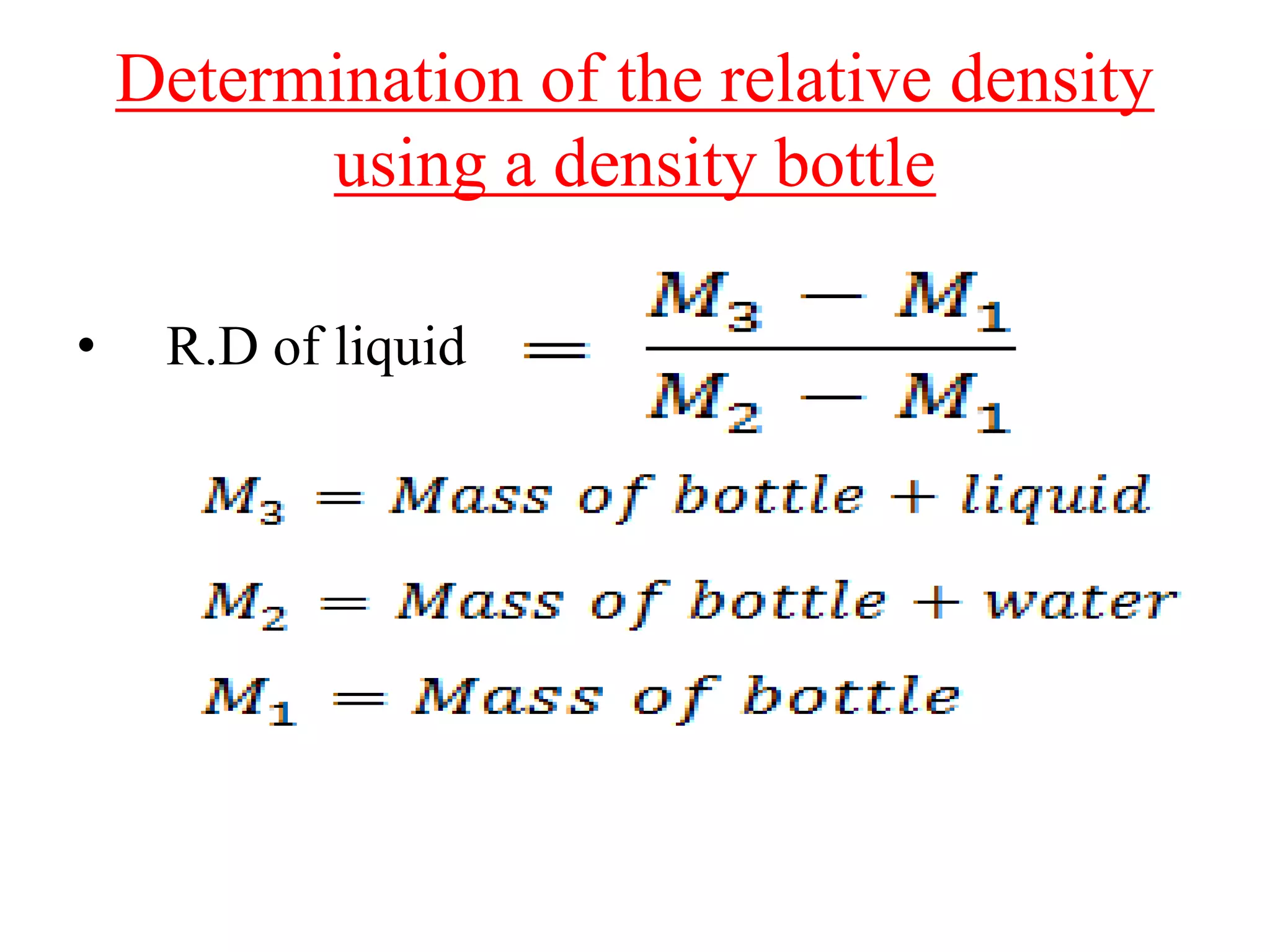
![NUMERICALS FROM CONCISE
PHYSICS
9. The mass of an empty density bottle is 21.8 g, when filled
completely with water it is 41.8 g and when filled completely
with liquid it is 40.6 g. Find:
(a) the volume of density bottle
(b) the relative density of liquid
Ans.(a) Mass of water having volume same as density bottle =
(M2 − M1) = (41.8 − 21.8) = 20 g
Volume of water = Volume of density bottle = (Mass of
water)/(Density of water in C.G.S unit) = 20 g/ (1 g/cm3) = 20
cm3 = 20 ml [ As, 1 cm3 = 1 ml]
(b) Mass of liquid having same volume as density bottle = (M3 −
M1) = (40.6 − 21.8) = 18.8 g
Relative Density = (M3 − M1)/ (M2 − M1) = 18.8/20.0 = 0.94](https://image.slidesharecdn.com/class-8physicalquantitymeasurement2-201107073556/75/CLASS-8-PHYSICAL-QUANTITY-MEASUREMENT-28-2048.jpg)

![11.The mass of an empty density bottle is 30 g, it is 75 g when
filled completely with water and 65 g when filled completely
with liquid. Find :
(a)Volume of density bottle
(b)Density of liquid, &
(c)Relative density
Ans. Mass of water having same volume as density bottle = (75
− 30) = 45 g
Volume of density bottle = Volume of water = Mass/ Density in
C.G.S unit = 45 g/1g/cm3 = 45 cm3 = 45 ml [As,1cm3=1 ml]
Mass of liquid having same volume as density bottle = (65 −
30) = 35 g
Density of liquid = Mass/ Volume of liquid =
Mass/ Volume of density bottle = 35/45 = 0.77 g/cm3
R.D = Density in C.G.S unit = 0.77](https://image.slidesharecdn.com/class-8physicalquantitymeasurement2-201107073556/75/CLASS-8-PHYSICAL-QUANTITY-MEASUREMENT-30-2048.jpg)