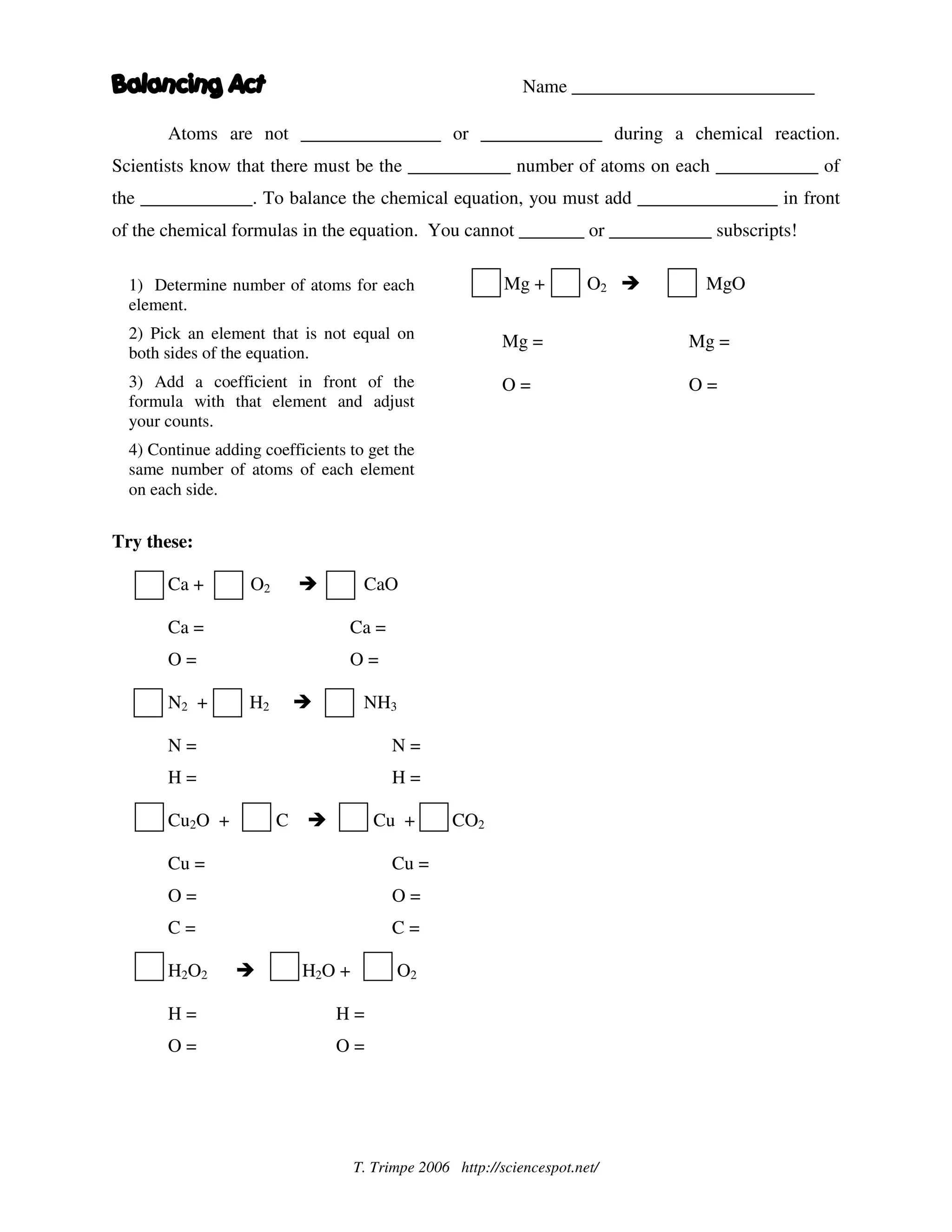
The document provides instructions for balancing chemical equations by ensuring the same number of atoms of each element are on both sides of the equation. It states that atoms are not created or destroyed in chemical reactions. To balance an equation, one must first determine the number of atoms of each element, then add coefficients in front of formulas as needed to make the atom counts equal on both sides. Coefficients cannot be added within formulas or change subscripts. An example problem is worked through step-by-step to demonstrate the process of balancing Mg + O2 → MgO.