This document summarizes several key water quality parameters:
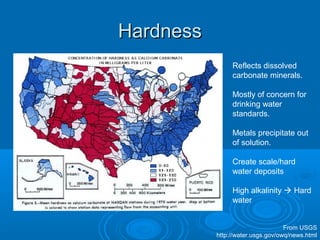
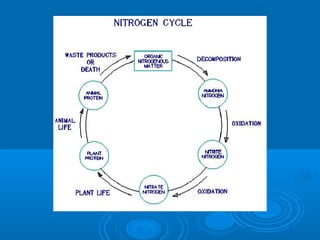
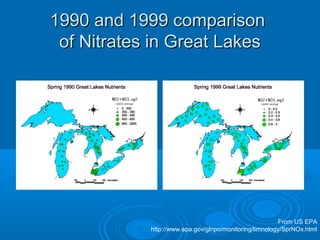
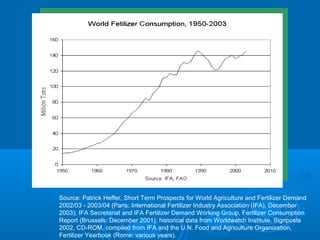
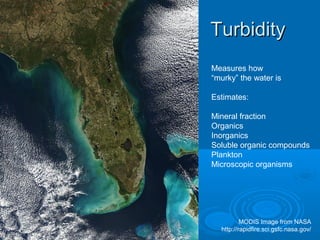
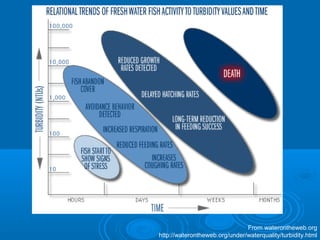
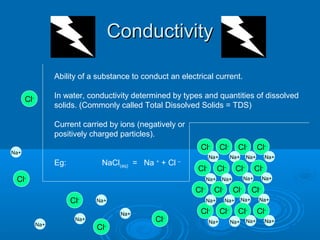
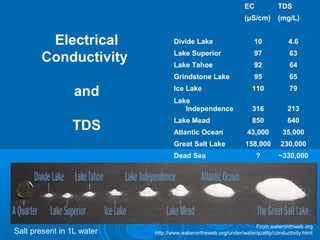
- Temperature, dissolved oxygen, pH, alkalinity, hardness, turbidity, conductivity, nitrates/phosphates are discussed in 1-2 sentences each. Hardness reflects dissolved minerals and is important for drinking water standards. Nitrates and phosphates occur naturally but can increase from fertilizers and cause eutrophication.
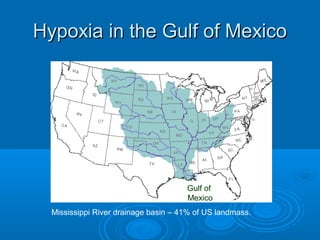
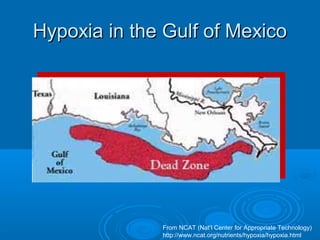
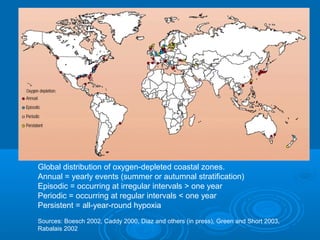
- Hypoxia in the Gulf of Mexico is discussed as being caused by excess nutrients from the Mississippi River drainage basin leading to algal blooms and oxygen depletion.
- Possible solutions mentioned include wetland restoration, reduced fertilizer use, and reductions in emissions and soil erosion.