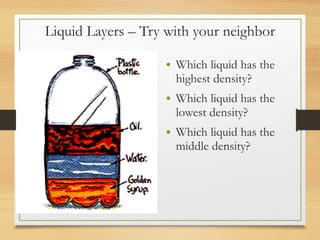
The document discusses density and how to calculate it. It provides examples of calculating the density of objects by measuring their mass and volume, such as a paper clip and eraser. It also discusses how liquids of different densities will separate into layers when mixed, with the highest density liquid sinking to the bottom. Students work through examples of calculating densities and identifying liquid layers.