This document provides instructions and information about upcoming assignments:
1) It lists materials needed like pencils and calculators.
2) It provides due dates for assignments on density, binder checks, and tests occurring on October 17th and 18th.
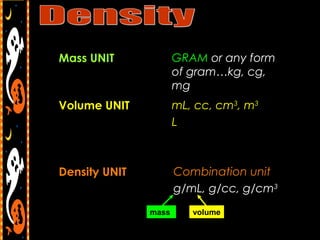
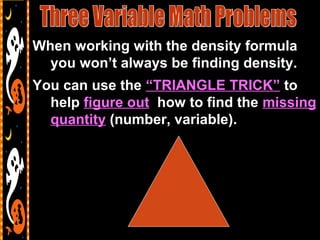
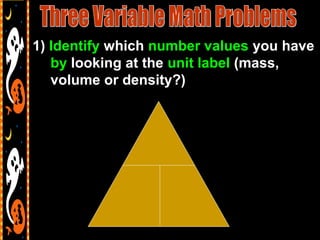
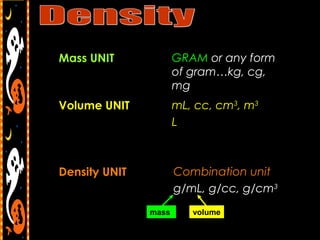
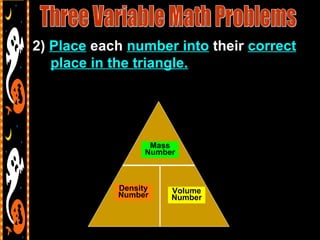
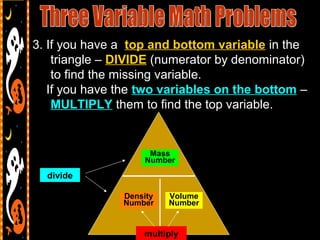
3) It also includes notes on density, describing it as a ratio of mass to volume, and how density can be used to determine if objects will sink or float in water. Formulas and examples of calculating density are presented.