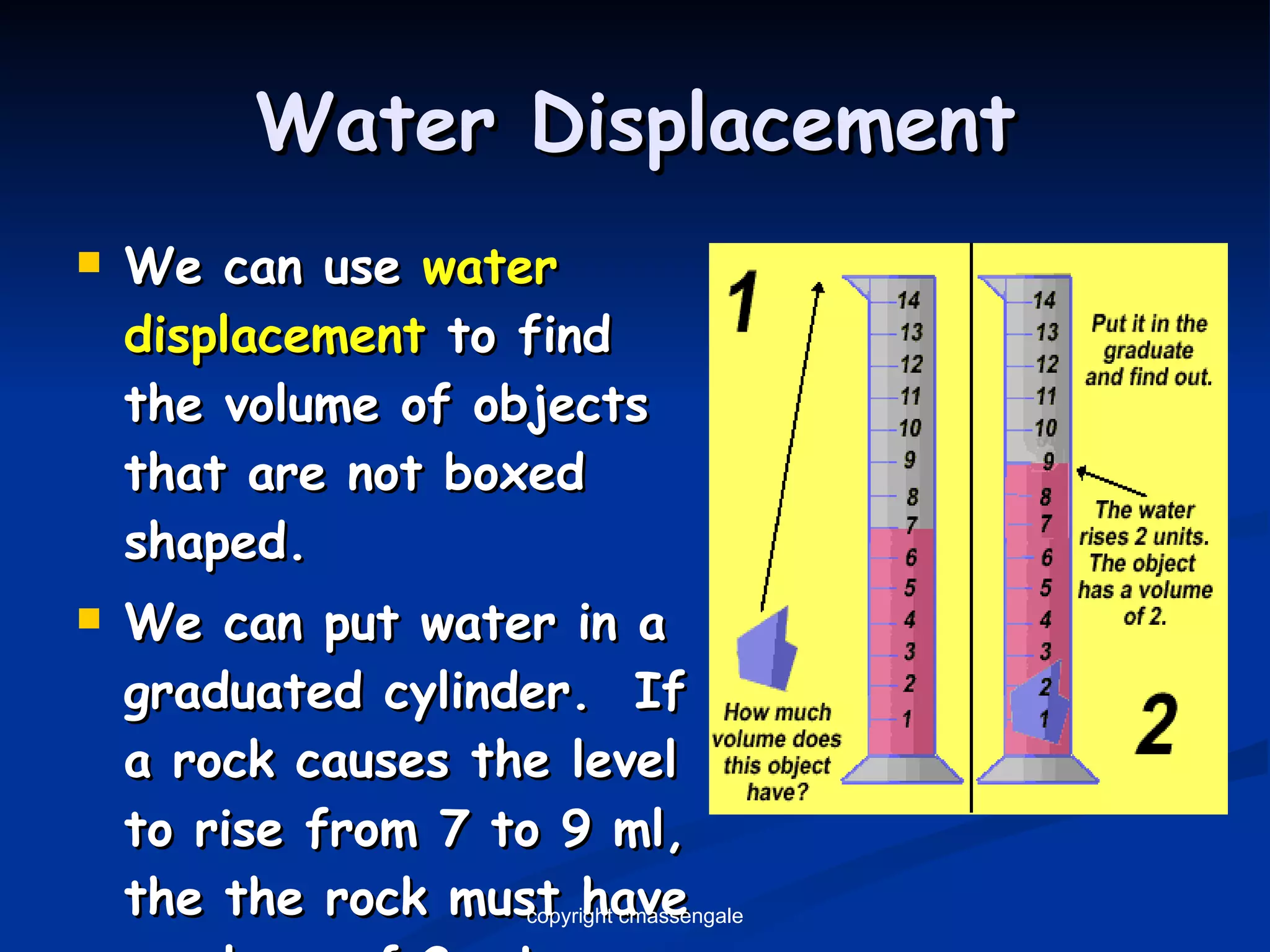
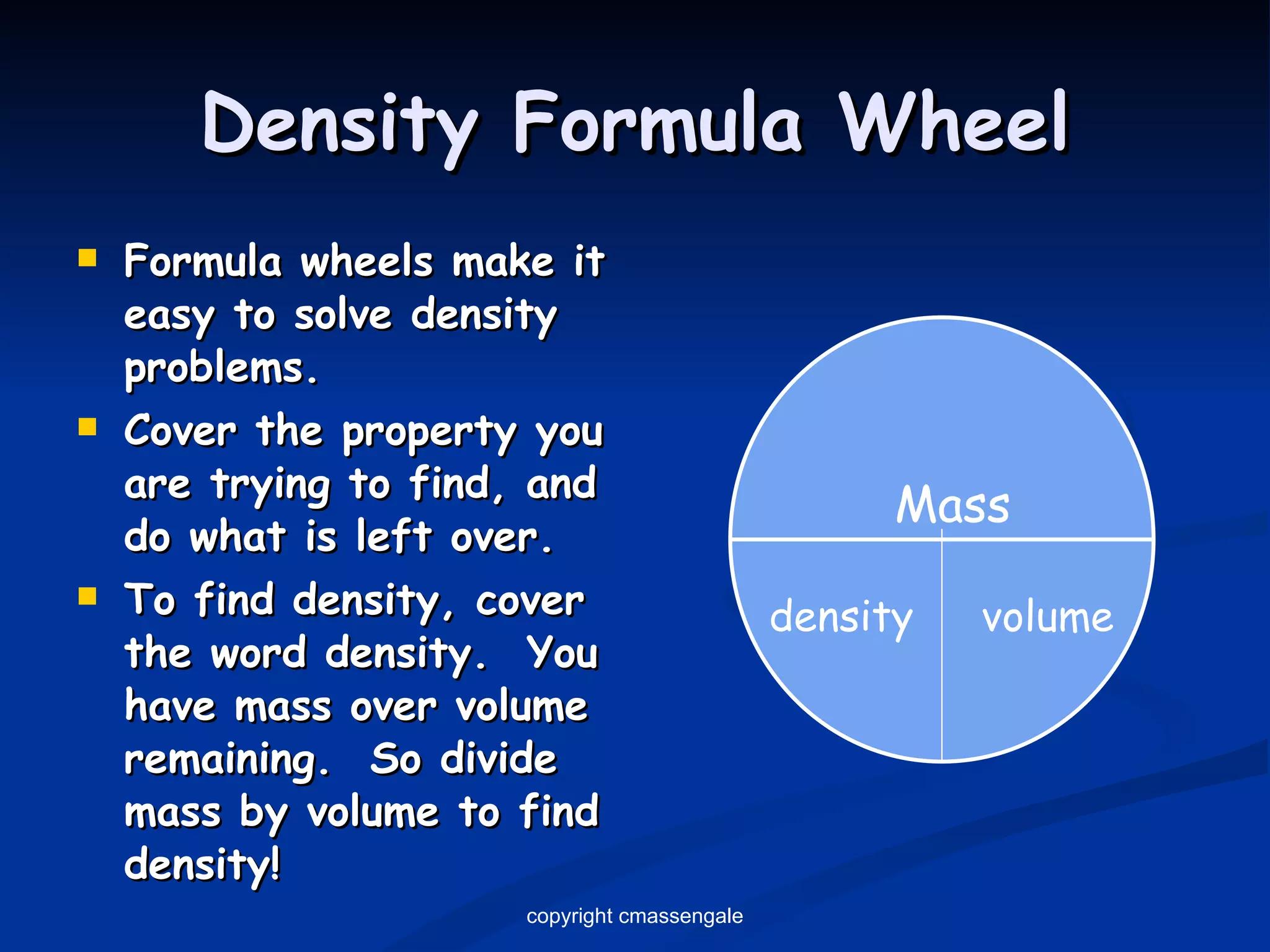
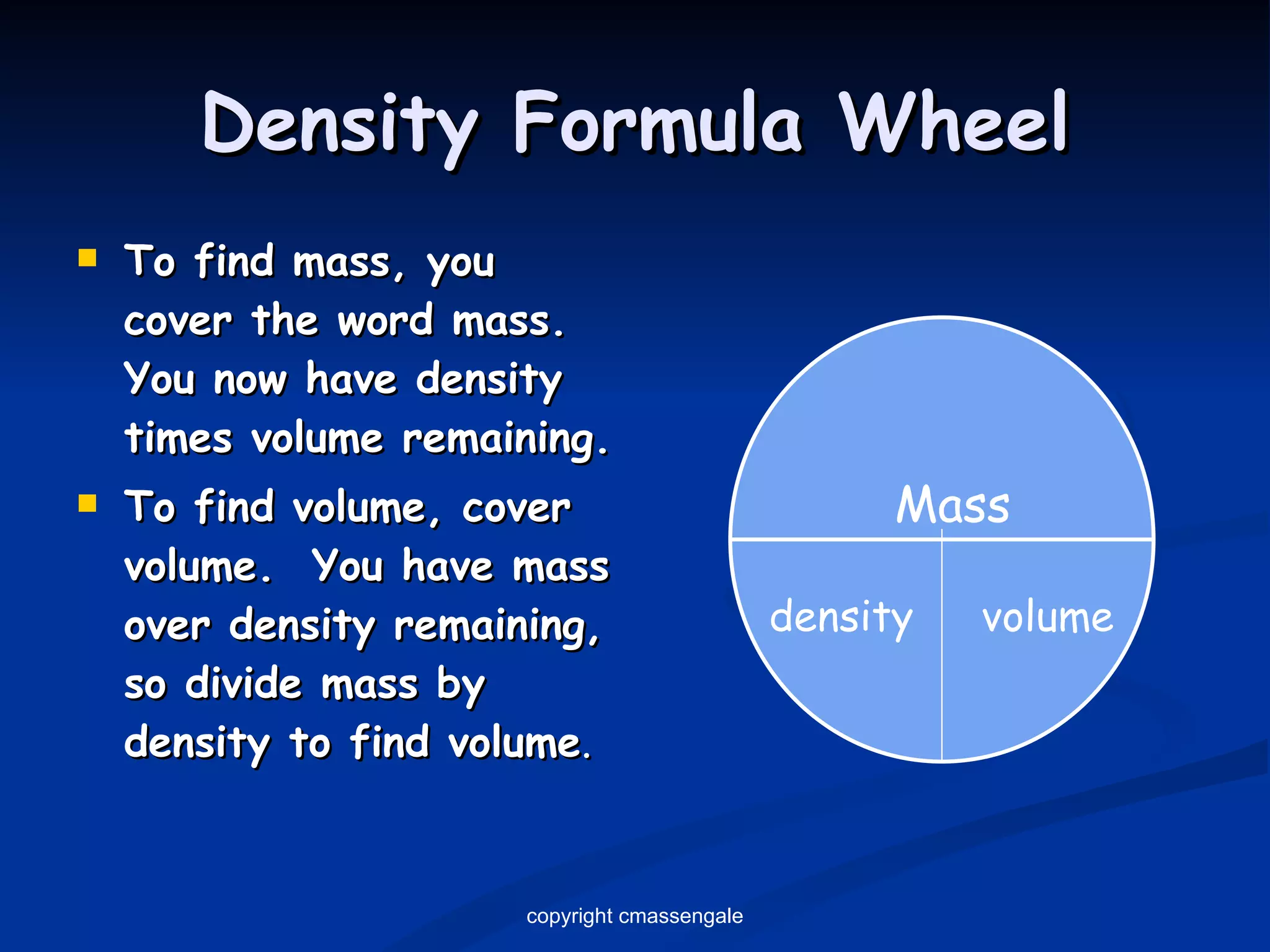
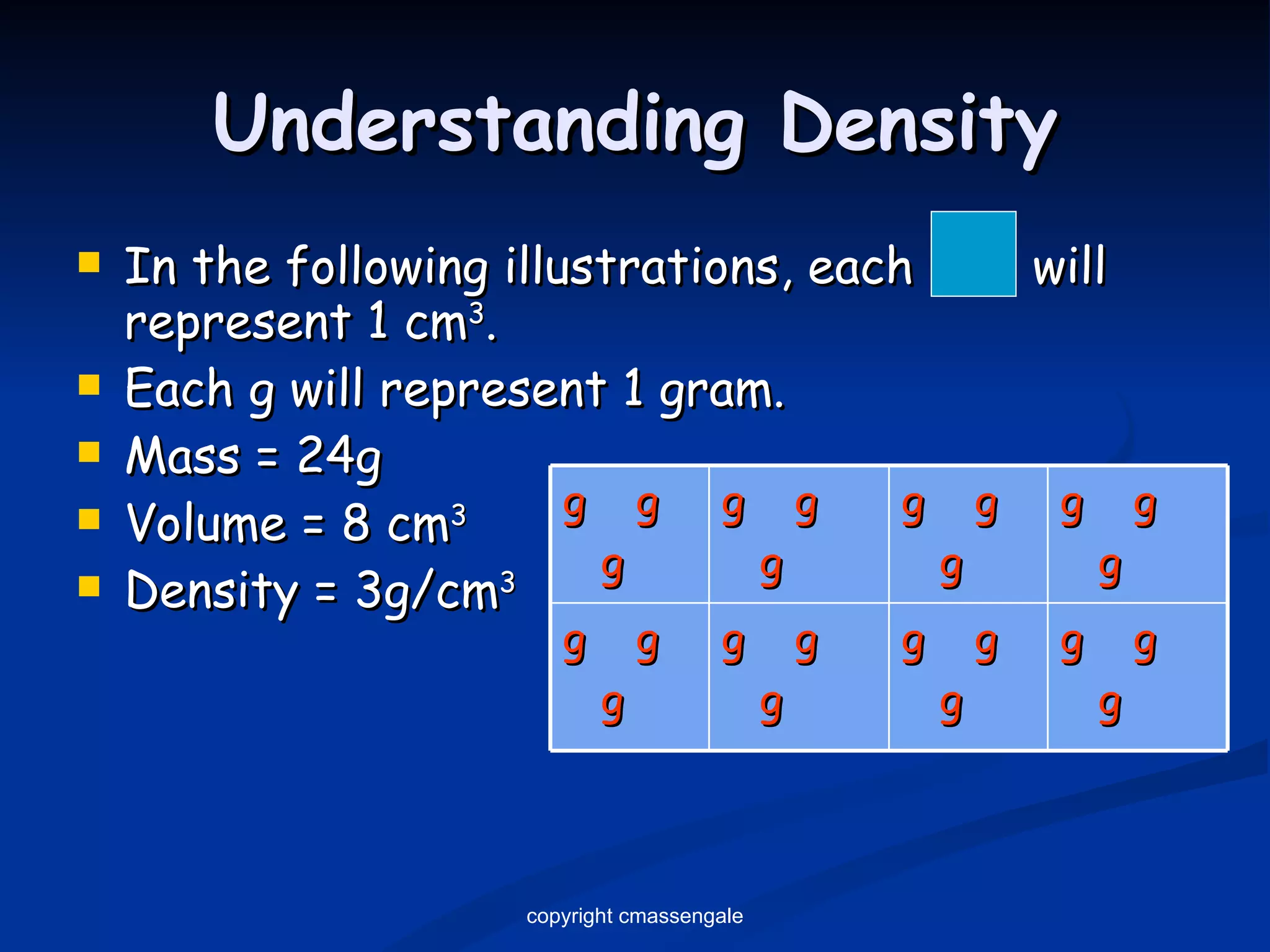
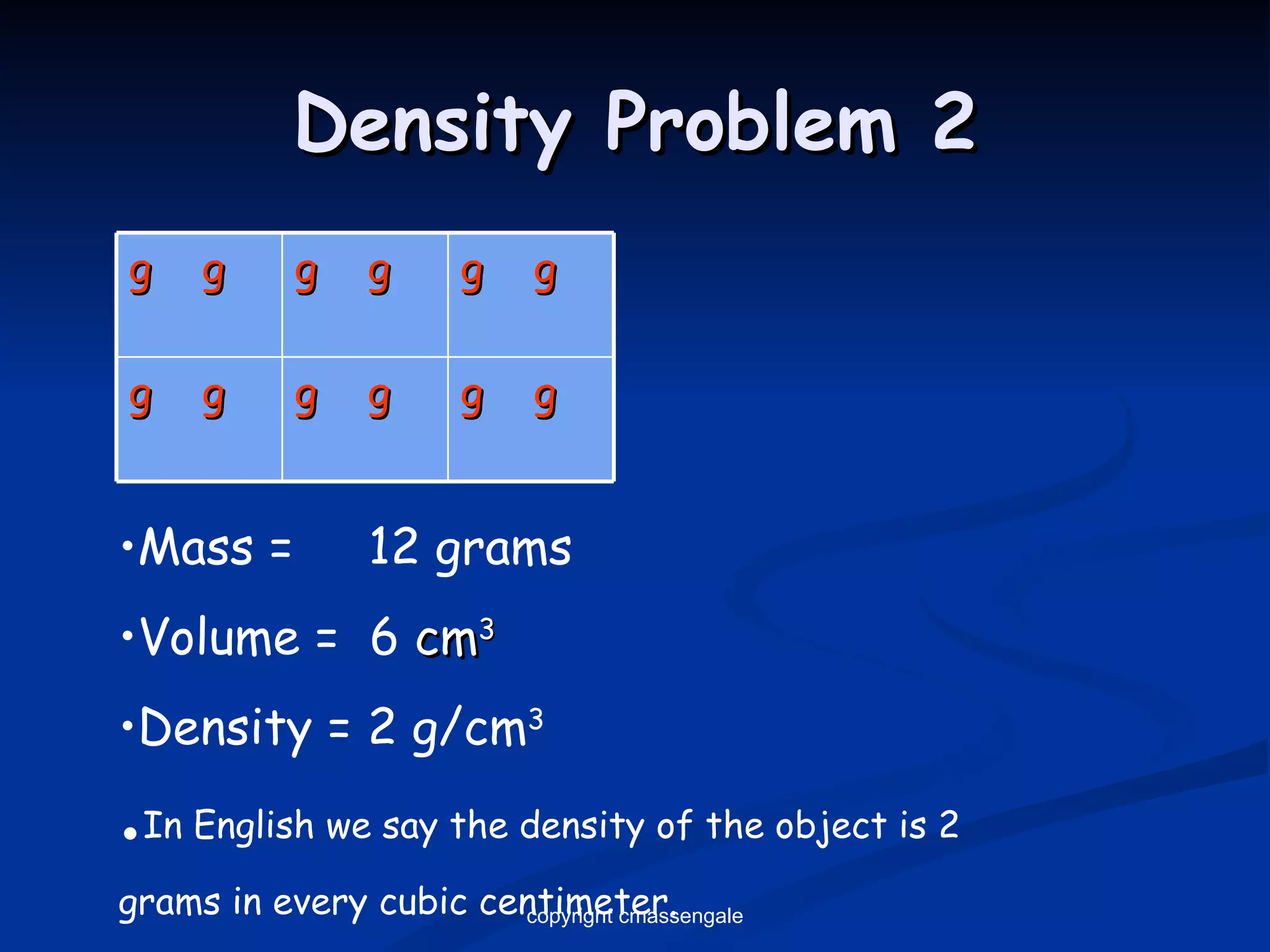
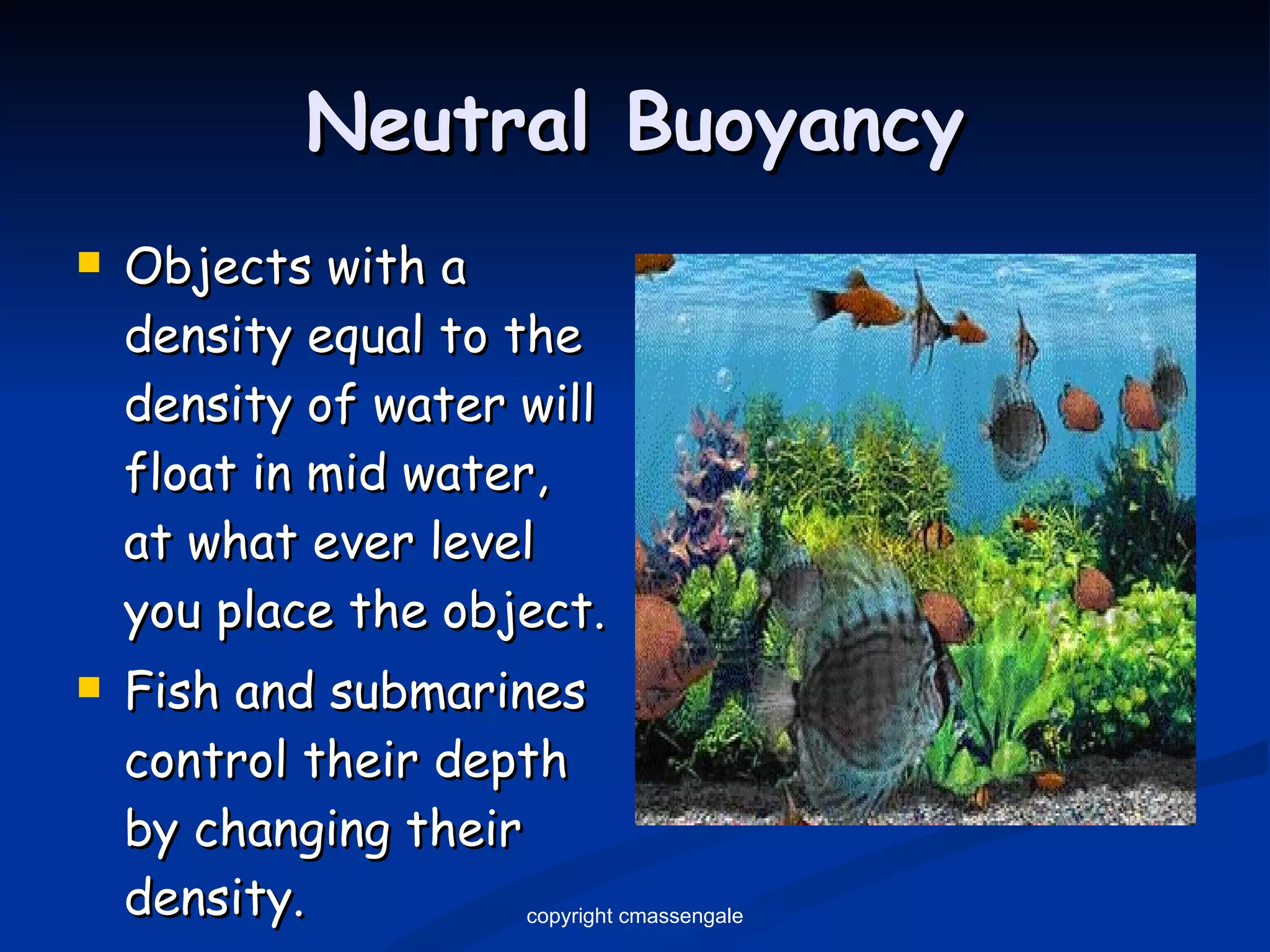
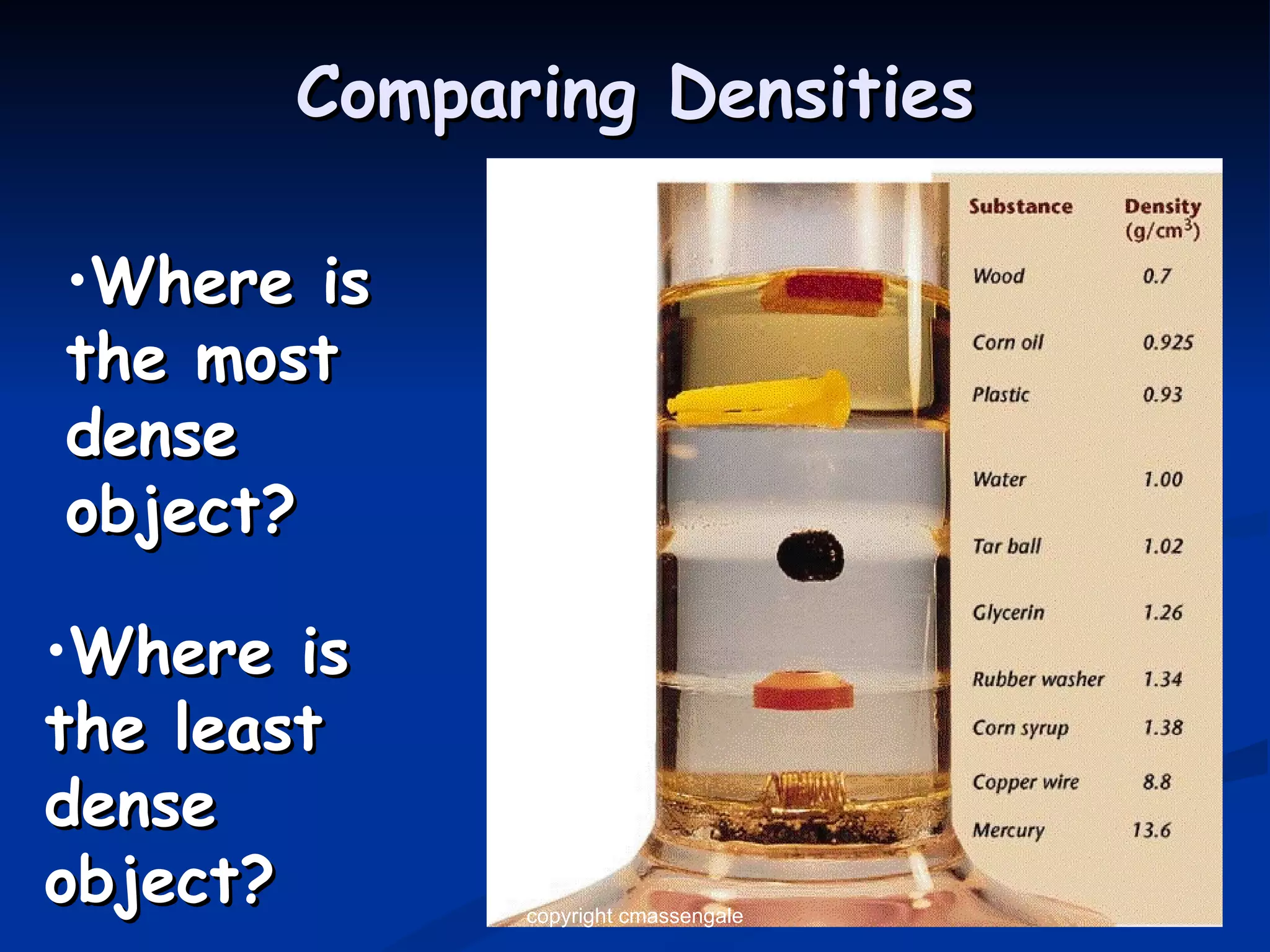
The document discusses the metric system and scientific measurements of length, mass, volume, density, and other key concepts:
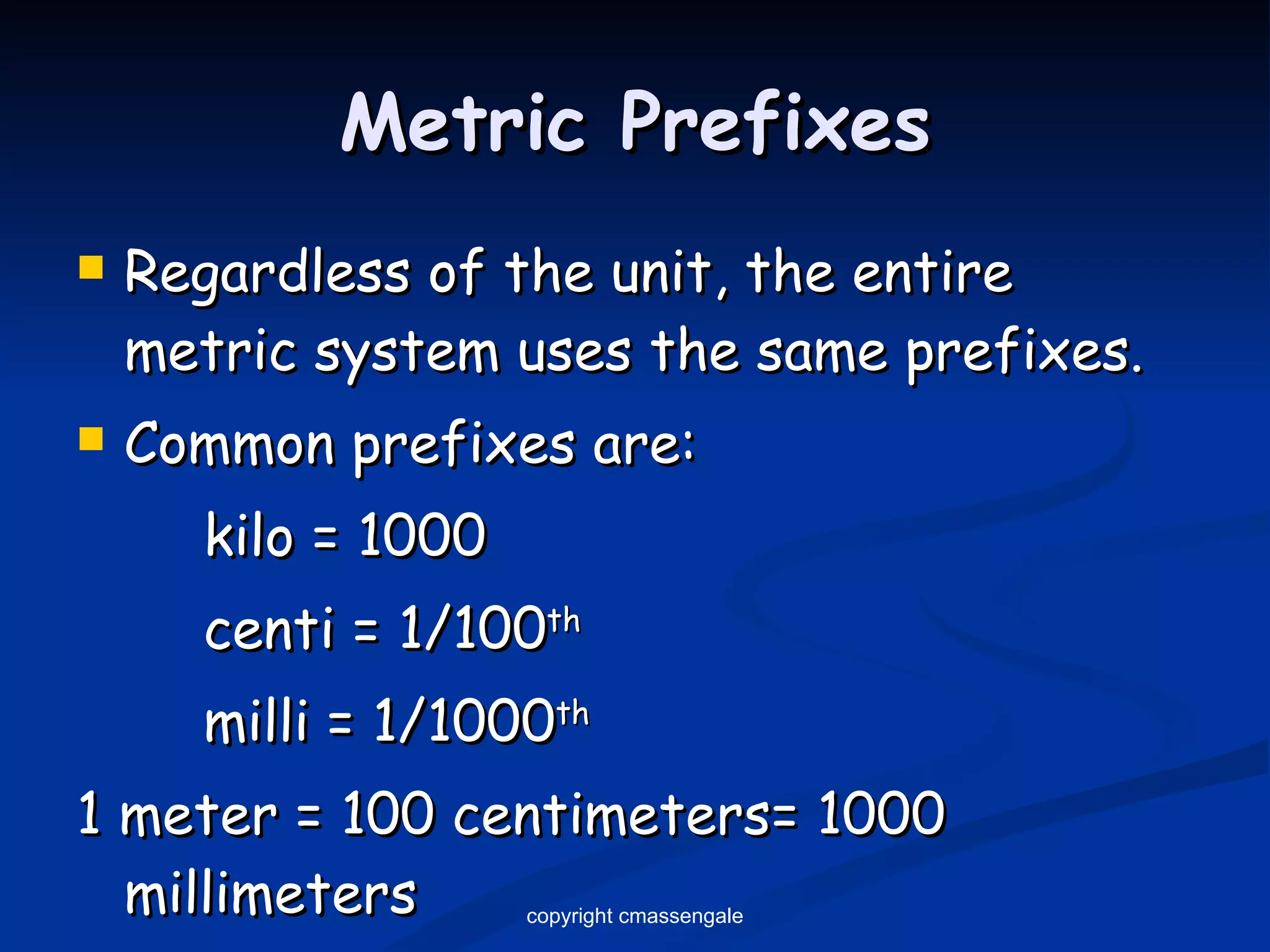
1) The metric system was developed in the late 1700s by the French and is based on powers of ten, making it easy to use. It is now used globally except in the United States.
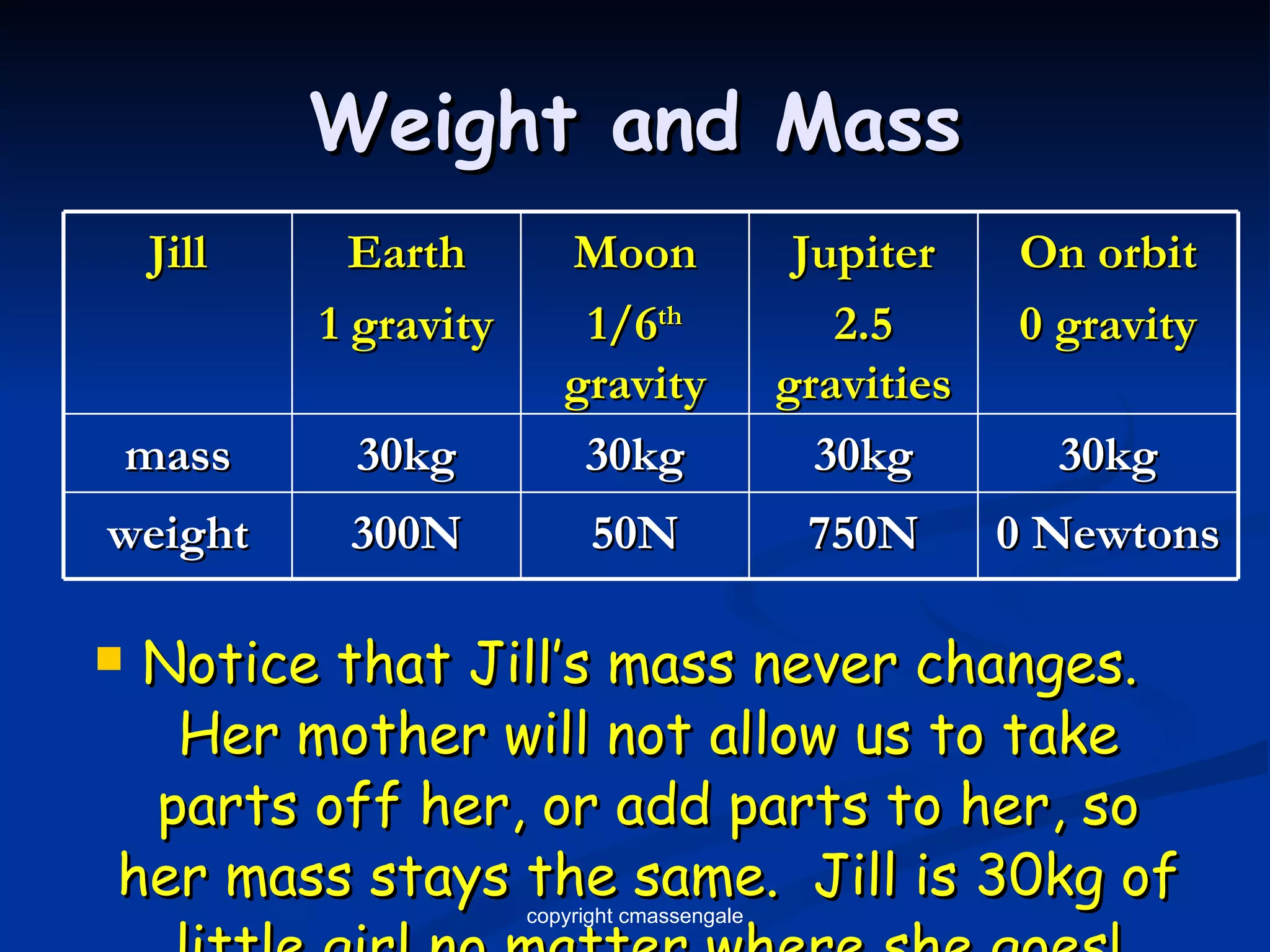
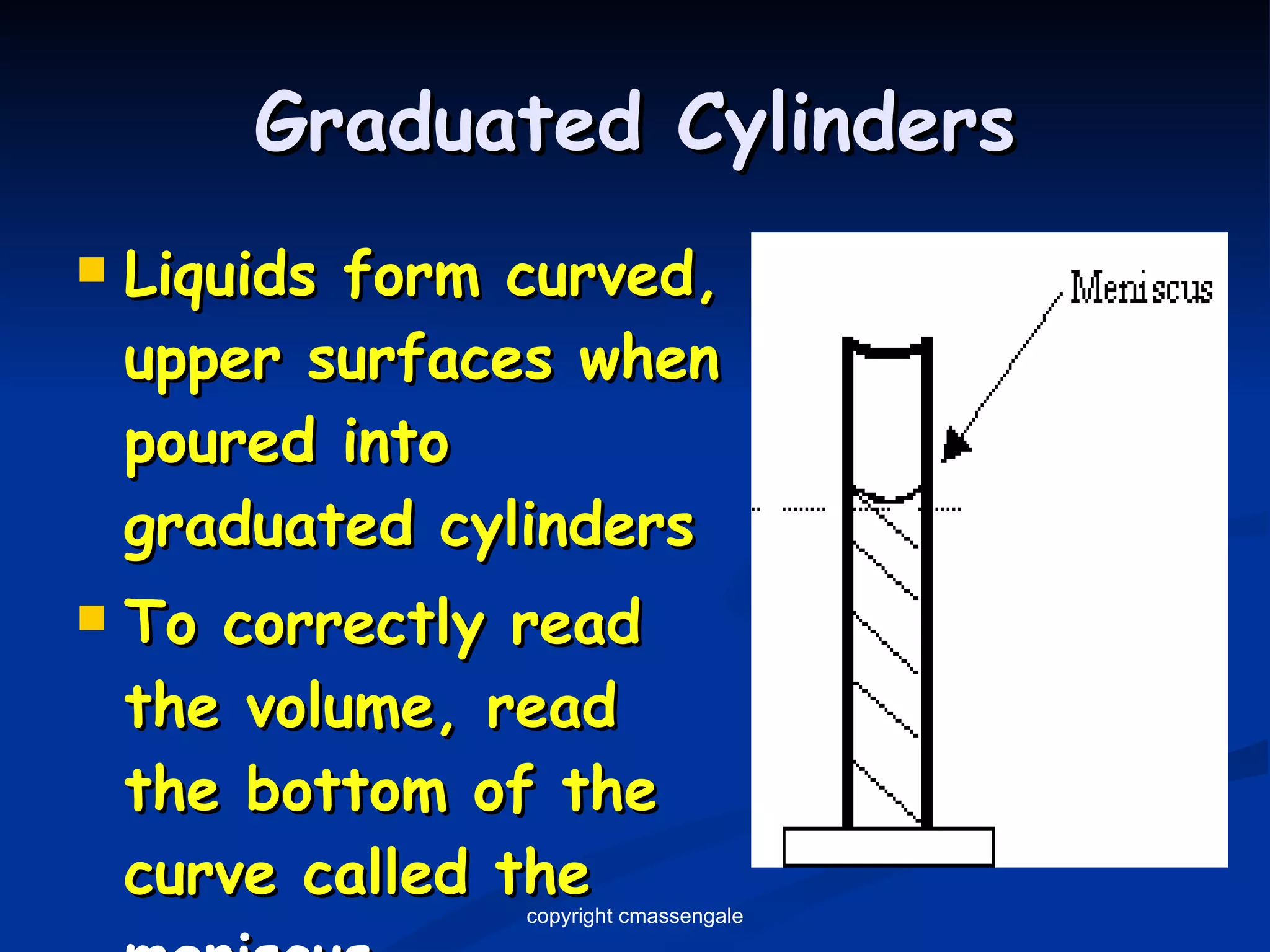
2) Key units include meters for length, grams for mass, and liters for volume. Density is a comparison of mass to volume.
3) The density of an object determines whether it will float or sink in water, with objects denser than water's 1 g/mL sinking and less dense objects floating.