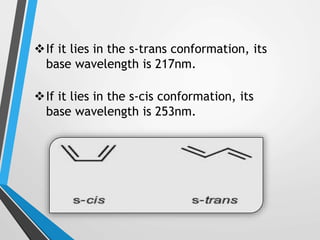
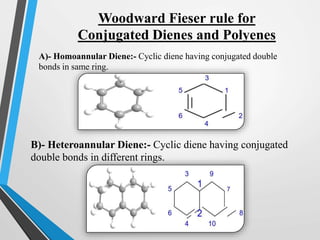
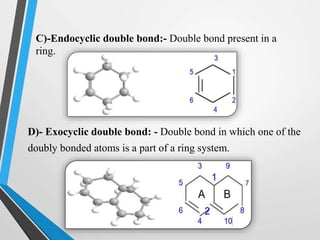
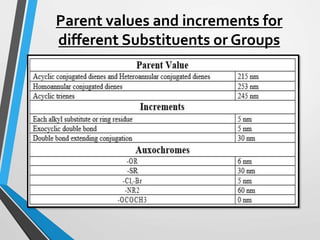
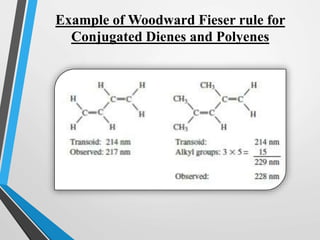
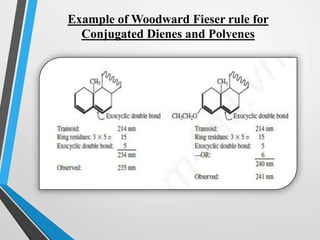
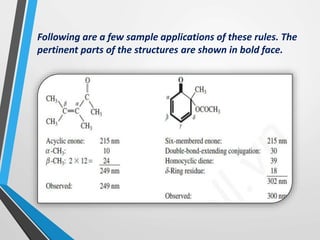
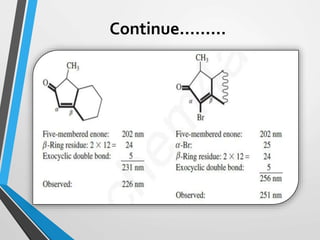
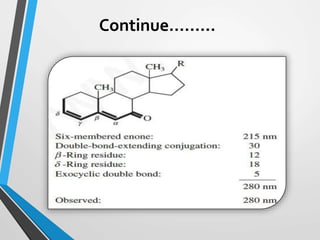
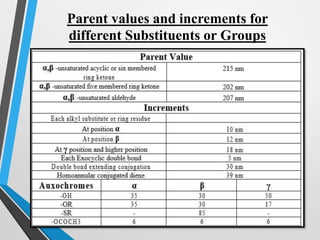
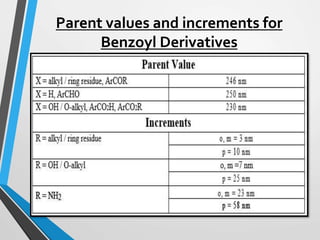
The document outlines the Woodward-Fieser rules developed for calculating the maximum wavelength (λmax) associated with molecular structures, with specific applications to conjugated dienes, α,β-unsaturated carbonyl compounds, and aromatic compounds. The rules provide a systematic method to determine λmax based on base values and substituent contributions. Additionally, it discusses various types of diene systems, their conformations, and how these factors influence UV absorption characteristics.