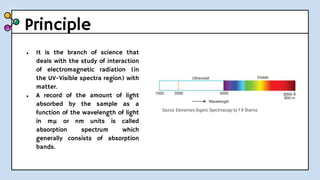
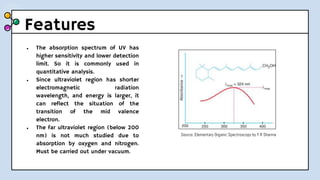
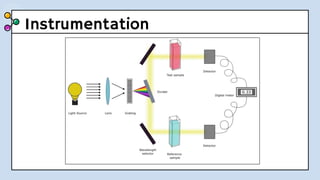
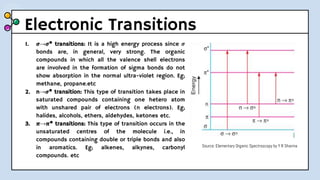
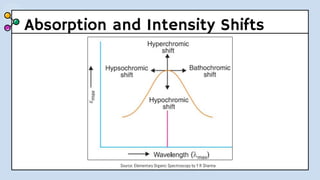
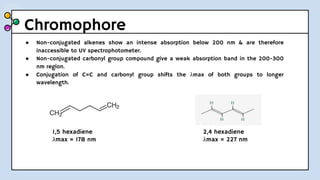
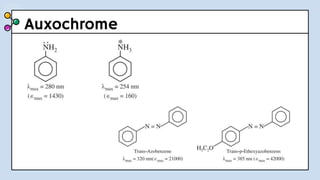
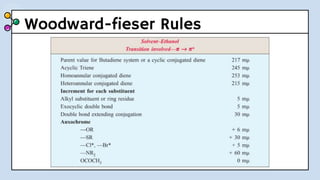
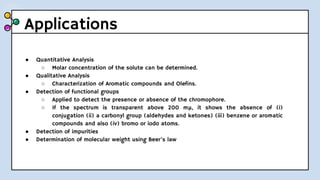
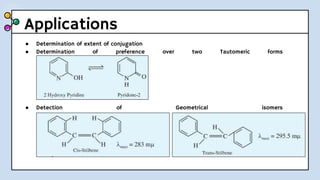
UV-Visible Spectroscopy involves the interaction of electromagnetic radiation in the ultraviolet and visible spectral regions with matter. When molecules absorb this radiation, electrons are excited from one energy level to a higher level. This causes absorption bands to appear in the absorption spectrum. Beer's law states that absorbance is directly proportional to concentration and path length. Chromophores are groups that absorb radiation, while auxochromes shift absorption to longer wavelengths by extending conjugation. Spectroscopy has applications in quantitative analysis, qualitative analysis, detection of functional groups and impurities, and determination of properties like extent of conjugation.