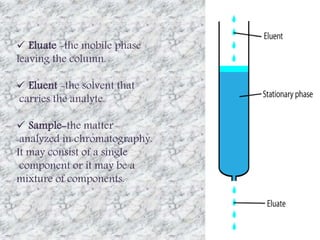
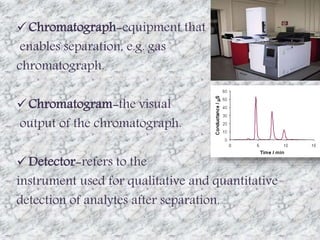
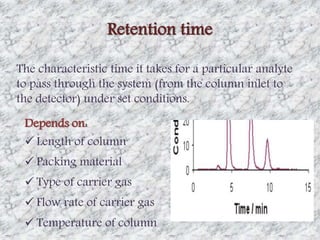
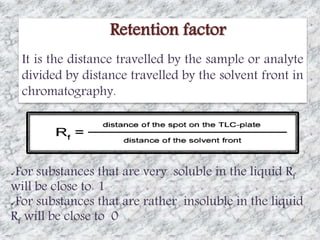
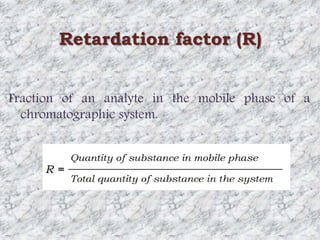
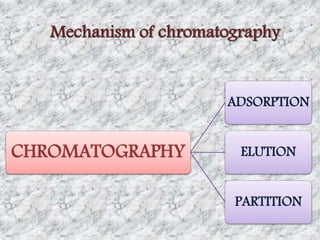
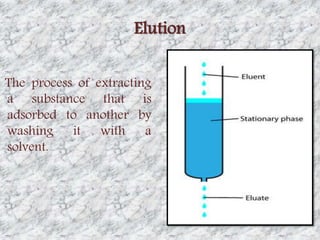
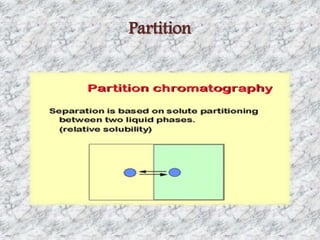
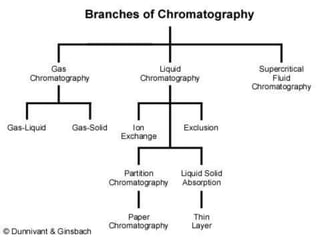
This document presents a group presentation on chromatography given to Sir M. Aamir. The presentation introduces chromatography as a technique for separating mixtures based on the distribution of components between a mobile and stationary phase. It discusses the invention of chromatography by Michael Tswett in 1901 and important milestones. The basic components, mechanisms, classifications, and common types of chromatography are described. Uses of chromatography in forensics, medicine, research, and the pharmaceutical industry are also mentioned.