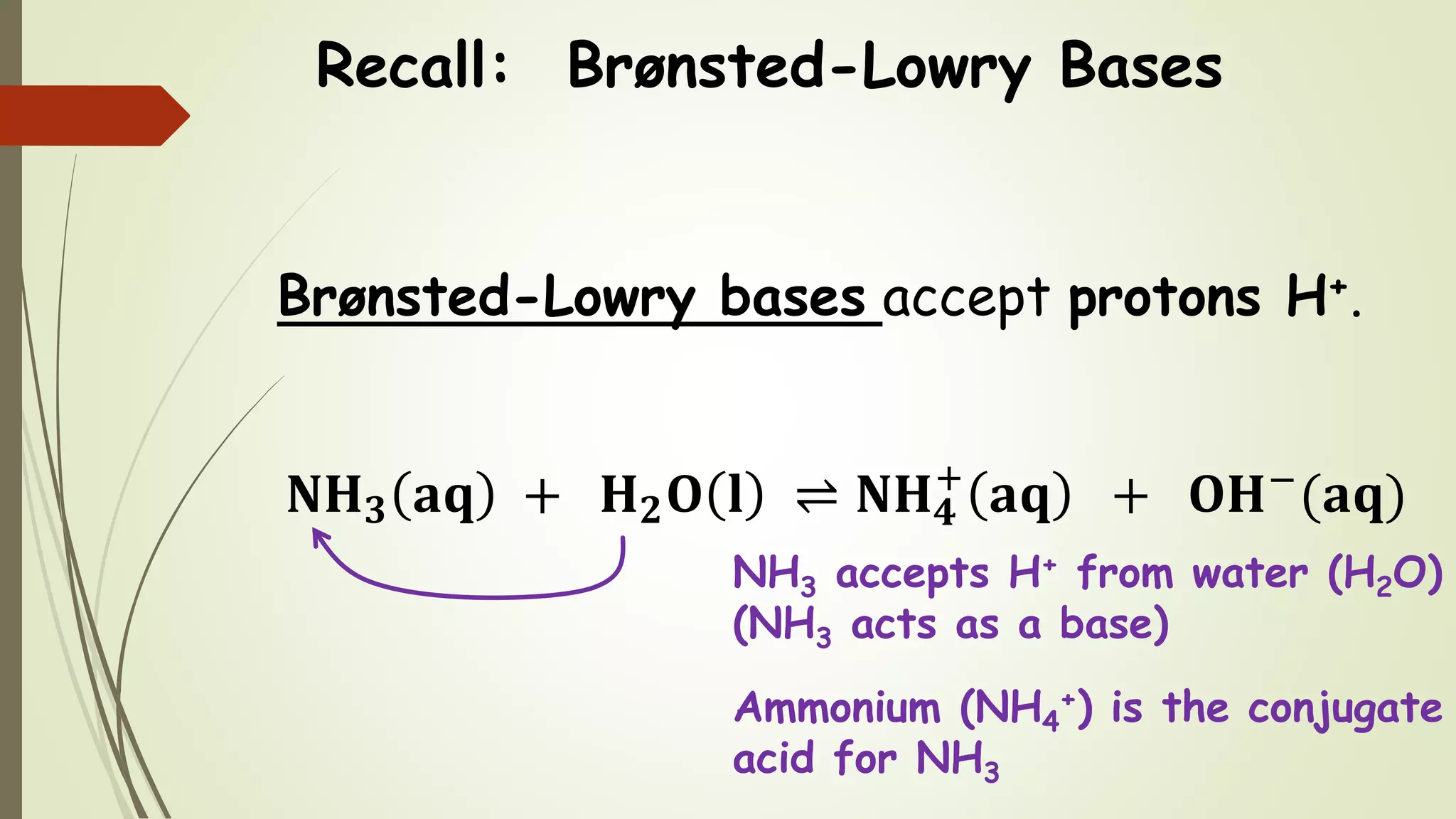
The document discusses acid-base equilibria, focusing on weak base equilibria and the calculation of pH and pOH for weak base solutions. It explains the differences between strong and weak bases, the Brønsted-Lowry definition, and how to use the base dissociation constant (Kb) to find the equilibrium concentrations in solutions. An illustrative example is provided using a 0.25 M NO2− solution with Kb = 2.2 × 10^-11, including step-by-step calculations of pH and pOH.






![ICE Tables, Kb, and Calculating pH for
a Weak Base Solution
Use Kb and an ICE table to determine the
[OH] at equilibrium. Next, calculate pOH
and use this value to calculate pH.
Calculate the pOH using the equilibrium [OH]
𝐍𝐎 𝟐
−
𝐚𝐪 + 𝐇 𝟐 𝐎 𝐥 ⇌ 𝐎𝐇−
𝐚𝐪 + 𝐇𝐍𝐎 𝟐(𝐚𝐪)
𝐊 𝐛 =
𝐎𝐇−
𝐇𝐍𝐎 𝟐
𝐍𝐎 𝟐
− = 𝟐. 𝟐 × 𝟏𝟎−𝟏𝟏](https://image.slidesharecdn.com/unit16weakbaseequilpt6final-160601181514/75/Chem-2-Acid-Base-Equilibria-VI-Weak-Base-Equilibria-and-Kb-Calculating-pH-and-pOH-for-a-Weak-Base-Solution-8-2048.jpg)









![Solving for x (assuming x is negligible)
𝟐. 𝟐 × 𝟏𝟎−𝟏𝟏
𝟎. 𝟐𝟓 = 𝐱 𝟐
𝟓. 𝟓 × 𝟏𝟎−𝟏𝟐
= 𝐱 𝟐
𝟓. 𝟓 × 𝟏𝟎−𝟏𝟐
𝟏
𝟐 = 𝐱 𝟐
𝟏
𝟐
𝟐. 𝟑𝟓 × 𝟏𝟎−𝟔
= 𝐱
x is the [OH]](https://image.slidesharecdn.com/unit16weakbaseequilpt6final-160601181514/75/Chem-2-Acid-Base-Equilibria-VI-Weak-Base-Equilibria-and-Kb-Calculating-pH-and-pOH-for-a-Weak-Base-Solution-18-2048.jpg)
![Calculate the pOH of the Weak Base
Solution
A 0.25 M NO2
solution is prepared. The Kb for NO2
is 2.2 10-11. Calculate the pH of this solution.
pOH = log [OH] = log [2.3510-6 ] = 5.63
We still need the pH!
0.25 2.3510-6
~ 0.25 M
2.3510-6 M
𝐍𝐎 𝟐
−
𝐚𝐪 + 𝐇 𝟐 𝐎 𝐥 ⇌ 𝐎𝐇− 𝐚𝐪 + 𝐇𝐍𝐎 𝟐(𝐚𝐪)
2.3510-6 M](https://image.slidesharecdn.com/unit16weakbaseequilpt6final-160601181514/75/Chem-2-Acid-Base-Equilibria-VI-Weak-Base-Equilibria-and-Kb-Calculating-pH-and-pOH-for-a-Weak-Base-Solution-19-2048.jpg)
![Recall: Relationships Between pH,
pOH, and pKw
pH + pOH = pKw
pKw = log [1.01014] = 14 (at 25C)
pH + pOH = 14 (at 25C)](https://image.slidesharecdn.com/unit16weakbaseequilpt6final-160601181514/75/Chem-2-Acid-Base-Equilibria-VI-Weak-Base-Equilibria-and-Kb-Calculating-pH-and-pOH-for-a-Weak-Base-Solution-20-2048.jpg)

