Embed presentation
Downloaded 212 times
![A. Ionization of Water [H 3 O + ][OH - ] = 1.0 10 -14 H 2 O + H 2 O H 3 O + + OH -](https://image.slidesharecdn.com/02phpres-110503073846-phpapp02/85/pH-presentation-2-320.jpg)
![A. Ionization of Water Find the hydroxide ion concentration of 3.0 10 -2 M HCl. [H 3 O + ][OH - ] = 1.0 10 -14 [3.0 10 -2 ][OH - ] = 1.0 10 -14 [OH - ] = 3.3 10 -13 M Acidic or basic? Acidic [H 3 O+] and [OH-] balance each other, more of one means less of the other](https://image.slidesharecdn.com/02phpres-110503073846-phpapp02/85/pH-presentation-3-320.jpg)
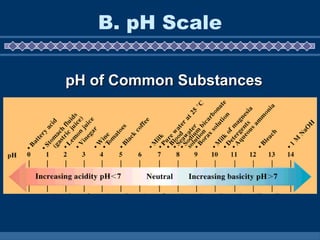
![B. pH Scale pH = -log[H 3 O + ] 0 7 INCREASING ACIDITY NEUTRAL INCREASING BASICITY 14 pouvoir hydrogène (Fr.) “ hydrogen power”](https://image.slidesharecdn.com/02phpres-110503073846-phpapp02/85/pH-presentation-4-320.jpg)
![B. pH Scale pH = -log[H 3 O + ] pOH = -log[OH - ] pH + pOH = 14](https://image.slidesharecdn.com/02phpres-110503073846-phpapp02/85/pH-presentation-6-320.jpg)
![B. pH Scale What is the pH of 0.050 M HNO 3 ? pH = -log[H 3 O + ] pH = -log[0.050] pH = 1.3 Acidic or basic? Acidic](https://image.slidesharecdn.com/02phpres-110503073846-phpapp02/85/pH-presentation-7-320.jpg)
![B. pH Scale What is the molarity of hydronium ions in a solution that has a pOH of 9.6? pH + pOH = 14 pH + 9.6 = 14 pH = 4.4 Acidic pH = -log[H 3 O + ] 4.4 = -log[H 3 O + ] -4.4 = log[H 3 O + ] [H 3 O + ] = 4.0 10 -5 M](https://image.slidesharecdn.com/02phpres-110503073846-phpapp02/85/pH-presentation-8-320.jpg)
This document discusses acids and bases, including the ionization of water and the pH scale. It defines pH as the negative logarithm of the hydronium ion concentration and explains how pH and pOH are related. Examples are provided to demonstrate how to calculate the hydroxide ion concentration from the hydronium ion concentration or vice versa using the pH scale.

![A. Ionization of Water [H 3 O + ][OH - ] = 1.0 10 -14 H 2 O + H 2 O H 3 O + + OH -](https://image.slidesharecdn.com/02phpres-110503073846-phpapp02/85/pH-presentation-2-320.jpg)
![A. Ionization of Water Find the hydroxide ion concentration of 3.0 10 -2 M HCl. [H 3 O + ][OH - ] = 1.0 10 -14 [3.0 10 -2 ][OH - ] = 1.0 10 -14 [OH - ] = 3.3 10 -13 M Acidic or basic? Acidic [H 3 O+] and [OH-] balance each other, more of one means less of the other](https://image.slidesharecdn.com/02phpres-110503073846-phpapp02/85/pH-presentation-3-320.jpg)
![B. pH Scale pH = -log[H 3 O + ] 0 7 INCREASING ACIDITY NEUTRAL INCREASING BASICITY 14 pouvoir hydrogène (Fr.) “ hydrogen power”](https://image.slidesharecdn.com/02phpres-110503073846-phpapp02/85/pH-presentation-4-320.jpg)

![B. pH Scale pH = -log[H 3 O + ] pOH = -log[OH - ] pH + pOH = 14](https://image.slidesharecdn.com/02phpres-110503073846-phpapp02/85/pH-presentation-6-320.jpg)
![B. pH Scale What is the pH of 0.050 M HNO 3 ? pH = -log[H 3 O + ] pH = -log[0.050] pH = 1.3 Acidic or basic? Acidic](https://image.slidesharecdn.com/02phpres-110503073846-phpapp02/85/pH-presentation-7-320.jpg)
![B. pH Scale What is the molarity of hydronium ions in a solution that has a pOH of 9.6? pH + pOH = 14 pH + 9.6 = 14 pH = 4.4 Acidic pH = -log[H 3 O + ] 4.4 = -log[H 3 O + ] -4.4 = log[H 3 O + ] [H 3 O + ] = 4.0 10 -5 M](https://image.slidesharecdn.com/02phpres-110503073846-phpapp02/85/pH-presentation-8-320.jpg)