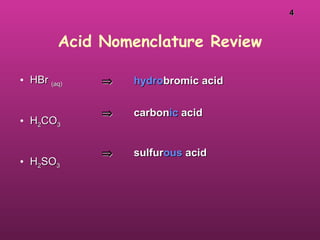
The document discusses the properties and definitions of acids and bases. It defines acids as substances that produce hydrogen (H+) ions or hydronium (H3O+) ions in water. Acids taste sour and react with metals and carbonates. Bases produce hydroxide (OH-) ions in water, taste bitter and slippery, and feel soapy. Common strong acids include HNO3, HCl, and H2SO4. Strong acids and bases ionize completely in water. Weak acids and bases only partially ionize. pH is a measure of hydrogen ion concentration in solutions. The autoionization of water and the pH scale are also explained.











![1313
Calculating the pH
pH = - log [H+]
(Remember that the [ ] mean Molarity)
Example: If [H+
] = 1 X 10-10
pH = - log 1 X 10-10
pH = - (- 10)
pH = 10
Example: If [H+
] = 1.8 X 10-5
pH = - log 1.8 X 10-5
pH = - (- 4.74)
pH = 4.74](https://image.slidesharecdn.com/acidsbases-180317101315/85/Acids-bases-13-320.jpg)
![1414
pH calculations – Solving for H+pH calculations – Solving for H+
If the pH of Coke is 3.12, [HIf the pH of Coke is 3.12, [H++
] = ???] = ???
Because pH = - log [HBecause pH = - log [H++
] then] then
- pH = log [H- pH = log [H++
]]
Take antilog (10Take antilog (10xx
) of both) of both
sides and getsides and get
1010-pH-pH
==[H[H++
]]
[H[H++
] = 10] = 10-3.12-3.12
= 7.6 x 10= 7.6 x 10-4-4
MM
*** to find antilog on your calculator, look for “Shift” or “2*** to find antilog on your calculator, look for “Shift” or “2ndnd
function” and then the log buttonfunction” and then the log button](https://image.slidesharecdn.com/acidsbases-180317101315/85/Acids-bases-14-320.jpg)
![1515
pH calculations – Solving for H+pH calculations – Solving for H+
• A solution has a pH of 8.5. What is theA solution has a pH of 8.5. What is the
Molarity of hydrogen ions in theMolarity of hydrogen ions in the
solution?solution?
pH = - log [HpH = - log [H++
]]
8.5 = - log [H8.5 = - log [H++
]]
-8.5 = log [H-8.5 = log [H++
]]
Antilog -8.5 = antilog (log [HAntilog -8.5 = antilog (log [H++
])])
1010-8.5-8.5
= [H= [H++
]]
3.16 X 103.16 X 10-9-9
= [H= [H++
]]](https://image.slidesharecdn.com/acidsbases-180317101315/85/Acids-bases-15-320.jpg)
![1616
More About Water
KKww = [H= [H33OO++
] [OH] [OH--
] = 1.00 x 10] = 1.00 x 10-14-14
at 25at 25 oo
CC
In aIn a neutralneutral solution [Hsolution [H33OO++
] = [OH] = [OH--
]]
so Kso Kww = [H= [H33OO++
]]22
= [OH= [OH--
]]22
and so [Hand so [H33OO++
] = [OH] = [OH--
] = 1.00 x 10] = 1.00 x 10-7-7
MM
AutoionizationAutoionization](https://image.slidesharecdn.com/acidsbases-180317101315/85/Acids-bases-16-320.jpg)
![1717
pOH
• Since acids and bases areSince acids and bases are
opposites, pH and pOH areopposites, pH and pOH are
opposites!opposites!
• pOH does not really exist, but it ispOH does not really exist, but it is
useful for changing bases to pH.useful for changing bases to pH.
• pOH looks at the perspective of apOH looks at the perspective of a
basebase
pOH = - log [OHpOH = - log [OH--
]]
Since pH and pOH are on oppositeSince pH and pOH are on opposite
ends,ends,
pH + pOH = 14pH + pOH = 14](https://image.slidesharecdn.com/acidsbases-180317101315/85/Acids-bases-17-320.jpg)
![1818
[H[H33OO++
], [OH], [OH--
] and pH] and pH
What is the pH of theWhat is the pH of the
0.0010 M NaOH solution?0.0010 M NaOH solution?
[OH-] = 0.0010 (or 1.0 X 10[OH-] = 0.0010 (or 1.0 X 10-3-3
M)M)
pOH = - log 0.0010pOH = - log 0.0010
pOH = 3pOH = 3
pH = 14 – 3 = 11pH = 14 – 3 = 11
OR KOR Kww = [H= [H33OO++
] [OH] [OH--
]]
[H[H3OO++
] = 1.0 x 10] = 1.0 x 10-11-11
MM
pH = - log (1.0 x 10pH = - log (1.0 x 10-11-11
) = 11.00) = 11.00](https://image.slidesharecdn.com/acidsbases-180317101315/85/Acids-bases-18-320.jpg)
![1919
[OH[OH--
]]
[H[H++
]] pOHpOH
pHpH 1010
-pOH
-pOH
1010
-pH
-pH
-Log[H
-Log[H
++]]
-Log[OH
Log[OH
--]]
14
- pO
H
14
- pO
H
14
- pH
14
- pH
1.0
x
10
1.0
x
10
-14
-14
[O
H
[O
H
-- ]]
1.0
x
10
1.0
x
10
-14
-14
[H[H
++ ]]](https://image.slidesharecdn.com/acidsbases-180317101315/85/Acids-bases-19-320.jpg)



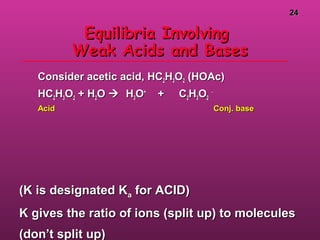
![2525
Equilibria Involving A Weak AcidEquilibria Involving A Weak Acid
You have 1.00 M HOAc. Calc. theYou have 1.00 M HOAc. Calc. the
equilibrium concs. of HOAc, Hequilibrium concs. of HOAc, H33OO++
, OAc, OAc--
,,
and the pH.and the pH.
Step 1.Step 1. Define equilibrium concs. in ICEDefine equilibrium concs. in ICE
table.table.
[HOAc][HOAc] [H[H33OO++
]][OAc[OAc--
]]
initialinitial
changechange
equilibequilib
1.001.00 00 00
-x-x +x+x +x+x
1.00-x1.00-x xx xx](https://image.slidesharecdn.com/acidsbases-180317101315/85/Acids-bases-25-320.jpg)

![2727
Equilibria Involving A Weak AcidEquilibria Involving A Weak Acid
Calculate the pH of a 0.0010 M solution ofCalculate the pH of a 0.0010 M solution of
formic acid, HCOformic acid, HCO22H.H.
HCOHCO22H + HH + H22OO HCOHCO22
--
+ H+ H33OO++
KKaa = 1.8 x 10= 1.8 x 10-4-4
Approximate solutionApproximate solution
[H[H33OO++
] = 4.2 x 10] = 4.2 x 10-4-4
M,M, pH = 3.37pH = 3.37
Exact SolutionExact Solution
[H[H33OO++
] = [HCO] = [HCO22
--
] = 3.4 x 10] = 3.4 x 10-4-4
MM
[HCO[HCO22H] = 0.0010 - 3.4 x 10H] = 0.0010 - 3.4 x 10-4-4
= 0.0007 M= 0.0007 M
pH = 3.47pH = 3.47](https://image.slidesharecdn.com/acidsbases-180317101315/85/Acids-bases-27-320.jpg)
![2828
Equilibria Involving A Weak BaseEquilibria Involving A Weak Base
You have 0.010 M NHYou have 0.010 M NH33. Calc. the pH.. Calc. the pH.
NHNH33 + H+ H22OO NHNH44
++
+ OH+ OH--
KKbb = 1.8 x 10= 1.8 x 10-5-5
Step 1.Step 1. Define equilibrium concs. in ICE tableDefine equilibrium concs. in ICE table
[NH[NH33]] [NH[NH44
++
]][OH[OH--
]]
initialinitial
changechange
equilibequilib
0.0100.010 00 00
-x-x +x+x +x+x
0.010 - x0.010 - x xx xx](https://image.slidesharecdn.com/acidsbases-180317101315/85/Acids-bases-28-320.jpg)
![2929
Equilibria Involving A Weak BaseEquilibria Involving A Weak Base
You have 0.010 M NHYou have 0.010 M NH33. Calc. the pH.. Calc. the pH.
NHNH33 + H+ H22OO NHNH44
++
+ OH+ OH--
KKbb = 1.8 x 10= 1.8 x 10-5-5
Step 1.Step 1. Define equilibrium concs. in ICE tableDefine equilibrium concs. in ICE table
[NH[NH33]] [NH[NH44
++
]][OH[OH--
]]
initialinitial
changechange
equilibequilib
0.0100.010 00 00
-x-x +x+x +x+x
0.010 - x0.010 - x xx xx](https://image.slidesharecdn.com/acidsbases-180317101315/85/Acids-bases-29-320.jpg)






