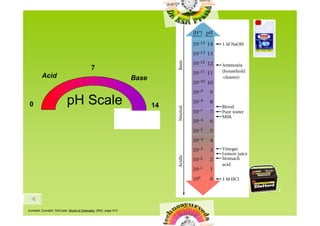
1) The pH scale is used to measure the strength of acids and bases, with pH defined as the negative log of the hydronium (H3O+) ion concentration.
2) Common substances span the pH scale from 0 to 14, with examples like stomach acid at pH 1 and bleach/drain cleaners at pH 12-13.
3) The pH of a solution depends on the relative concentrations of H3O+ and OH- ions, with acidic solutions having higher H3O+ and basic solutions having higher OH- concentrations.
4) Water self-ionizes according to the equilibrium H2O + H2O

![pH Scale
• We use this scale to measure the strength
of an acid or base.
• pH is defined as the –log[H+]
• pH can use the concentration of
hydronium ions or hydrogen ions.](https://image.slidesharecdn.com/phscaleandcalculations-091107004803-phpapp02/85/P-H-Scale-And-Calculations-2-320.jpg)
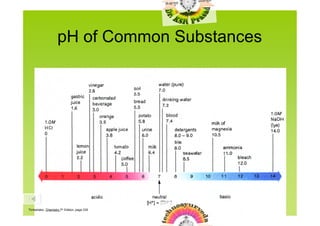
![pH of Common Substance
pH [H1+] [OH1-] pOH
14 1 x 10-14 1 x 10-0 0
NaOH, 0.1 M 13 1 x 10-13 1 x 10-1 1
Household bleach
12 1 x 10-12 1 x 10-2 2
More basic
Household ammonia
Lime water 11 1 x 10-11 1 x 10-3 3
Milk of magnesia
10 1 x 10-10 1 x 10-4 4
Borax
9 1 x 10-9 1 x 10-5 5
Baking soda
Egg white, seawater 8 1 x 10-8 1 x 10-6 6
Human blood, tears
Milk 7 1 x 10-7 1 x 10-7 7
Saliva
Rain 6 1 x 10-6 1 x 10-8 8
More acidic
Black coffee 5 1 x 10-5 1 x 10-9 9
Banana
Tomatoes 4 1 x 10-4 1 x 10-10 10
Wine
Cola, vinegar 3 1 x 10-3 1 x 10-11 11
Lemon juice
2 1 x 10-2 1 x 10-12 12
Gastric juice
1 1 x 10-1 1 x 10-13 13
0 1 x 100 1 x 10-14 14](https://image.slidesharecdn.com/phscaleandcalculations-091107004803-phpapp02/85/P-H-Scale-And-Calculations-5-320.jpg)
![Acid – Base Concentrations
10-1
pH = 3 pH = 11
concentration (moles/L)
H3O+ OH-
pH = 7
10-7
H3O+ OH-
OH- H3O+
10-14
[H3O+] > [OH-] [H3O+] = [OH-] [H3O+] < [OH-]
acidic neutral basic
Timberlake, Chemistry 7th Edition, page 332 solution solution solution](https://image.slidesharecdn.com/phscaleandcalculations-091107004803-phpapp02/85/P-H-Scale-And-Calculations-6-320.jpg)
![pH
pH = -log [H+]
Kelter, Carr, Scott, Chemistry A World of Choices 1999, page 285](https://image.slidesharecdn.com/phscaleandcalculations-091107004803-phpapp02/85/P-H-Scale-And-Calculations-7-320.jpg)
![Self-Ionization Of Water
• Even the purest of water conducts electricity. This
is due to the fact that water self-ionizes, that is, it
creates a small amount of H3O+ and OH-.
H2O + H2O H3O+ + OH-
Kw = [H3O+][OH-]
• Kw - ion product of water
Kw = 1.0 x 10-14 at 25 oC
• This equilibrium constant is very important because
it applies to all aqueous solutions - acids, bases,
salts, and non-electrolytes - not just to pure water.](https://image.slidesharecdn.com/phscaleandcalculations-091107004803-phpapp02/85/P-H-Scale-And-Calculations-8-320.jpg)
![Self ionization reaction of water:
+ -
O O O
H + H H H + O
H H
H H
2 H2O H3 O OH
K w [H3O ] [OH ] 10 -14
(at 25
C)
Kw
[H 3O ]
[OH-]](https://image.slidesharecdn.com/phscaleandcalculations-091107004803-phpapp02/85/P-H-Scale-And-Calculations-9-320.jpg)
![pH and pOH
• pH = - log[H3O+] [H3O+] = 10-pH
pOH = - log[OH-] [OH-] = 10-pOH
• pKw = pH + pOH = 14.00
• neutral solution: [H3O+] = [OH-] = 10 –7 M pH = 7.0
acidic solution: [H3O+] > 10-7 M pH < 7.0
basic solution: [H3O+] < 10-7 M pH > 7.0](https://image.slidesharecdn.com/phscaleandcalculations-091107004803-phpapp02/85/P-H-Scale-And-Calculations-10-320.jpg)
![Practice
• Finish the following for homework
Page 566 #12-15 (using Kw)
Page 569 # 16-19 (using pH = -log[H+]
Page 572 # 20-23
Page 578 # 2, 6-8](https://image.slidesharecdn.com/phscaleandcalculations-091107004803-phpapp02/85/P-H-Scale-And-Calculations-11-320.jpg)