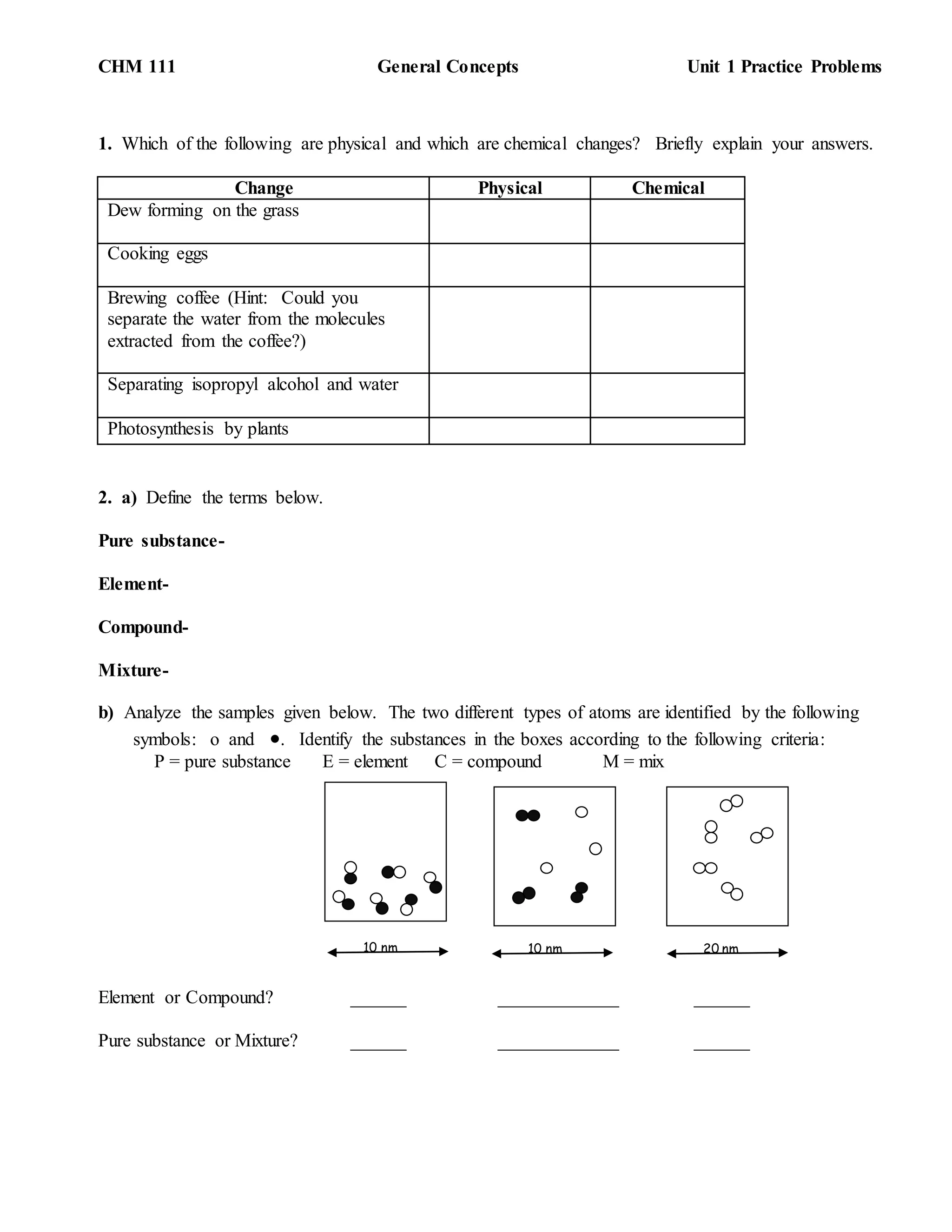
This document provides practice problems for a general chemistry unit covering concepts such as physical and chemical changes, pure substances and mixtures, separation techniques, properties, and density. It includes questions asking students to define terms, analyze samples, identify properties, describe separation methods, design experiments, and perform calculations.