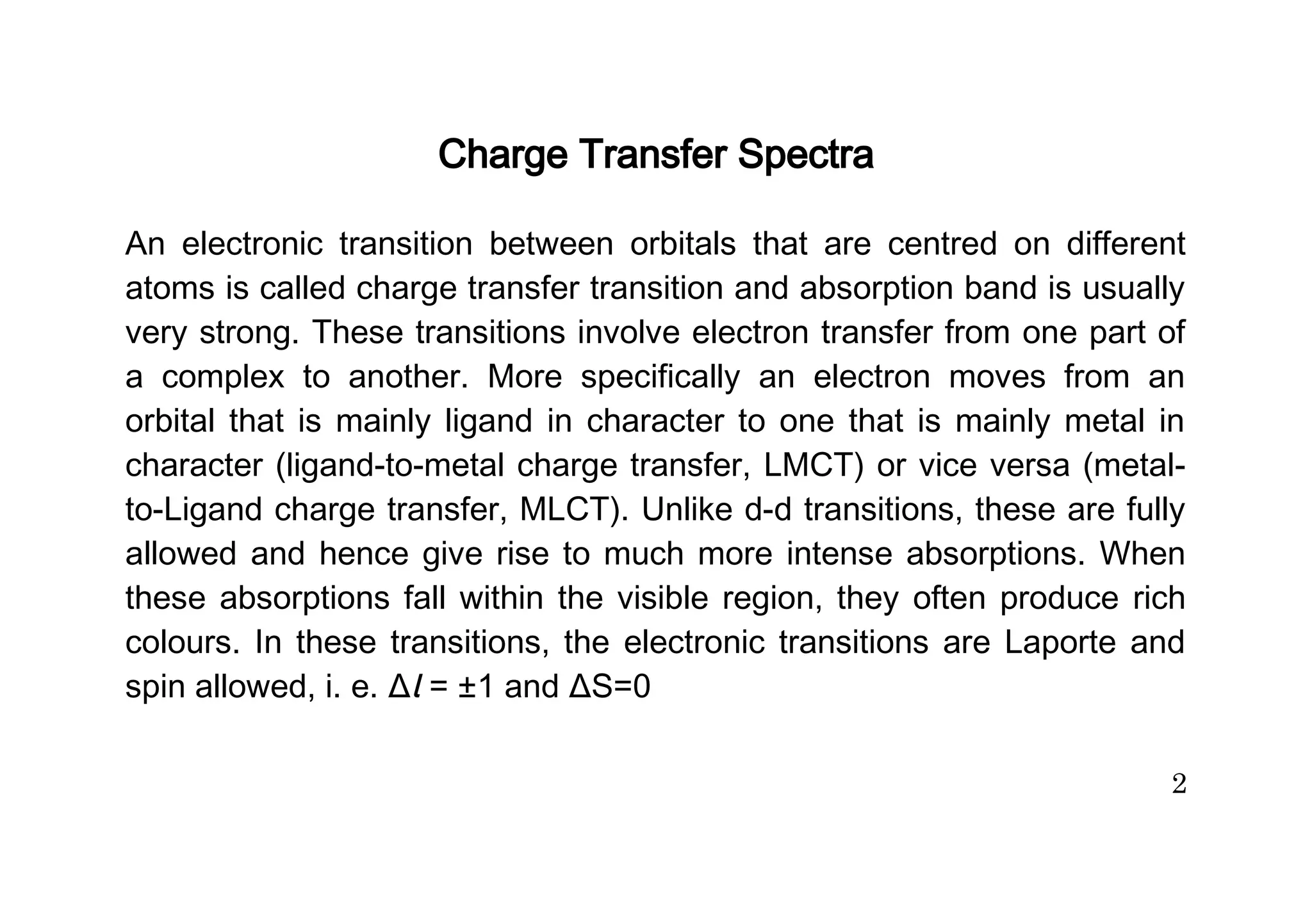
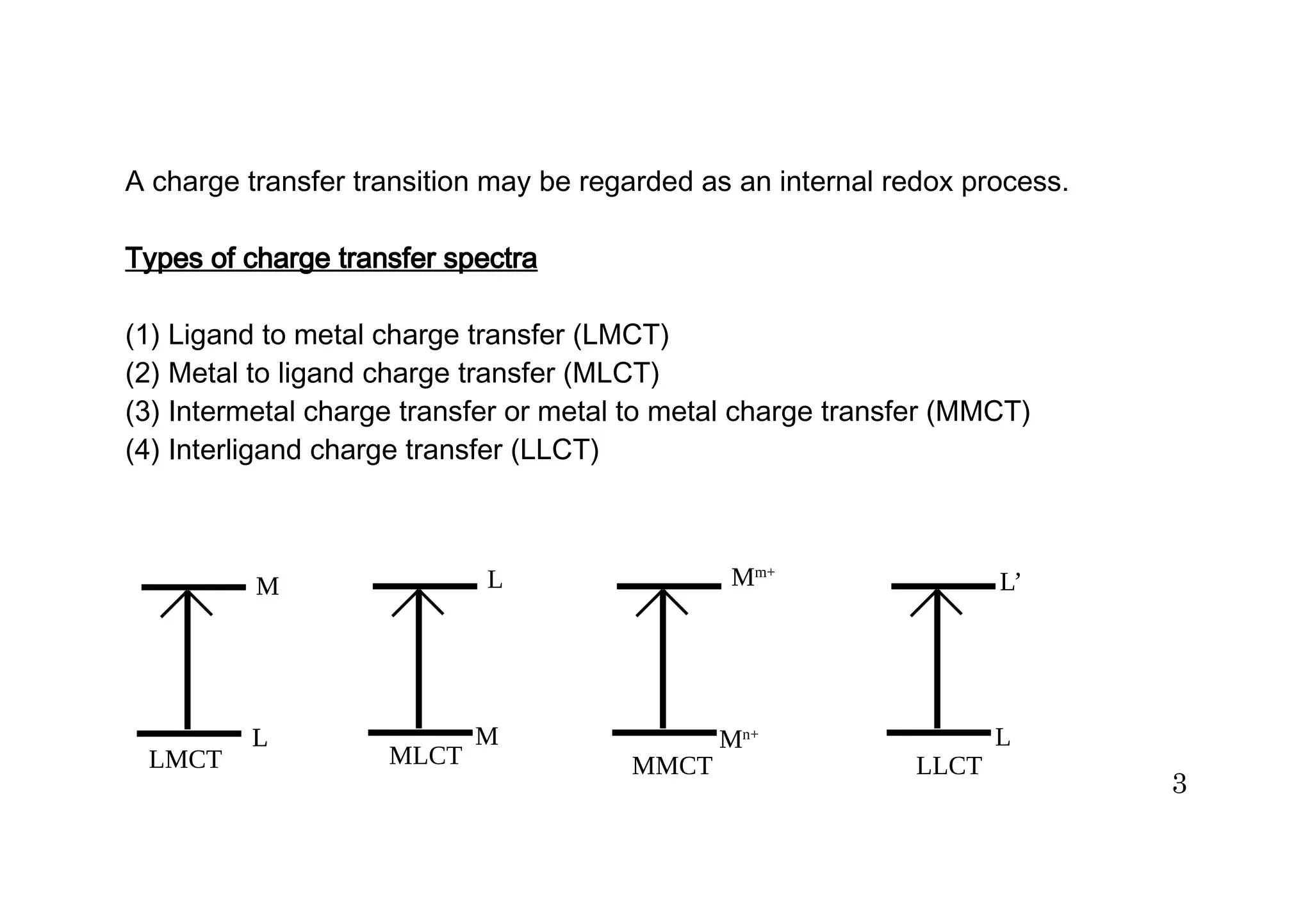
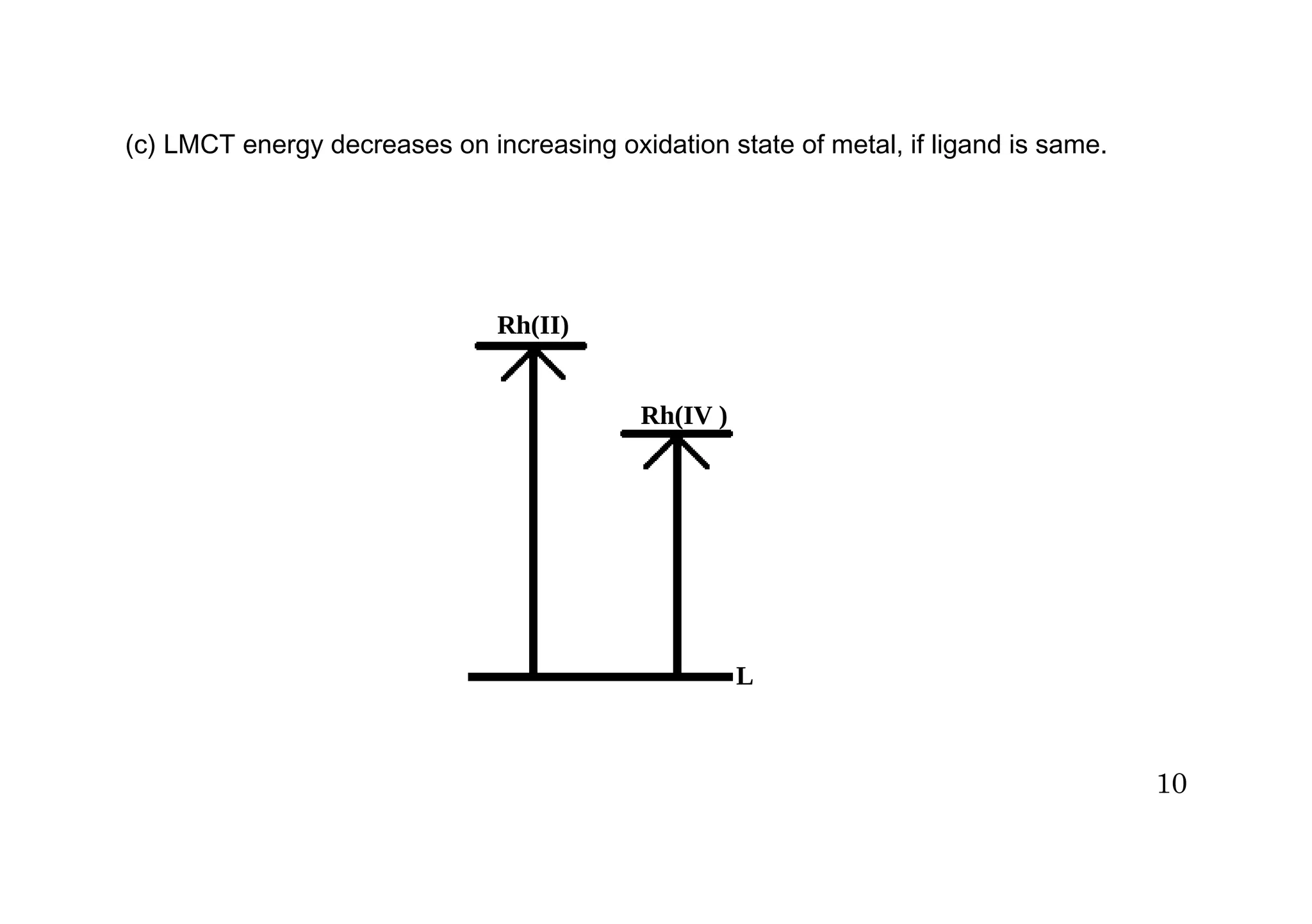
The document discusses charge transfer spectra in metal complexes. There are four main types of charge transfer transitions: ligand to metal (LMCT), metal to ligand (MLCT), intermetal or metal to metal (MMCT), and interligand (LLCT). LMCT involves electron transfer from ligand orbitals to metal orbitals, while MLCT is the reverse with electron transfer from metal to ligand orbitals. MMCT occurs between different oxidation states of the same metal. LLCT takes place between different ligands, one acting as an electron donor and the other as an acceptor. Examples are provided of each type of charge transfer and how they influence the color of complexes.










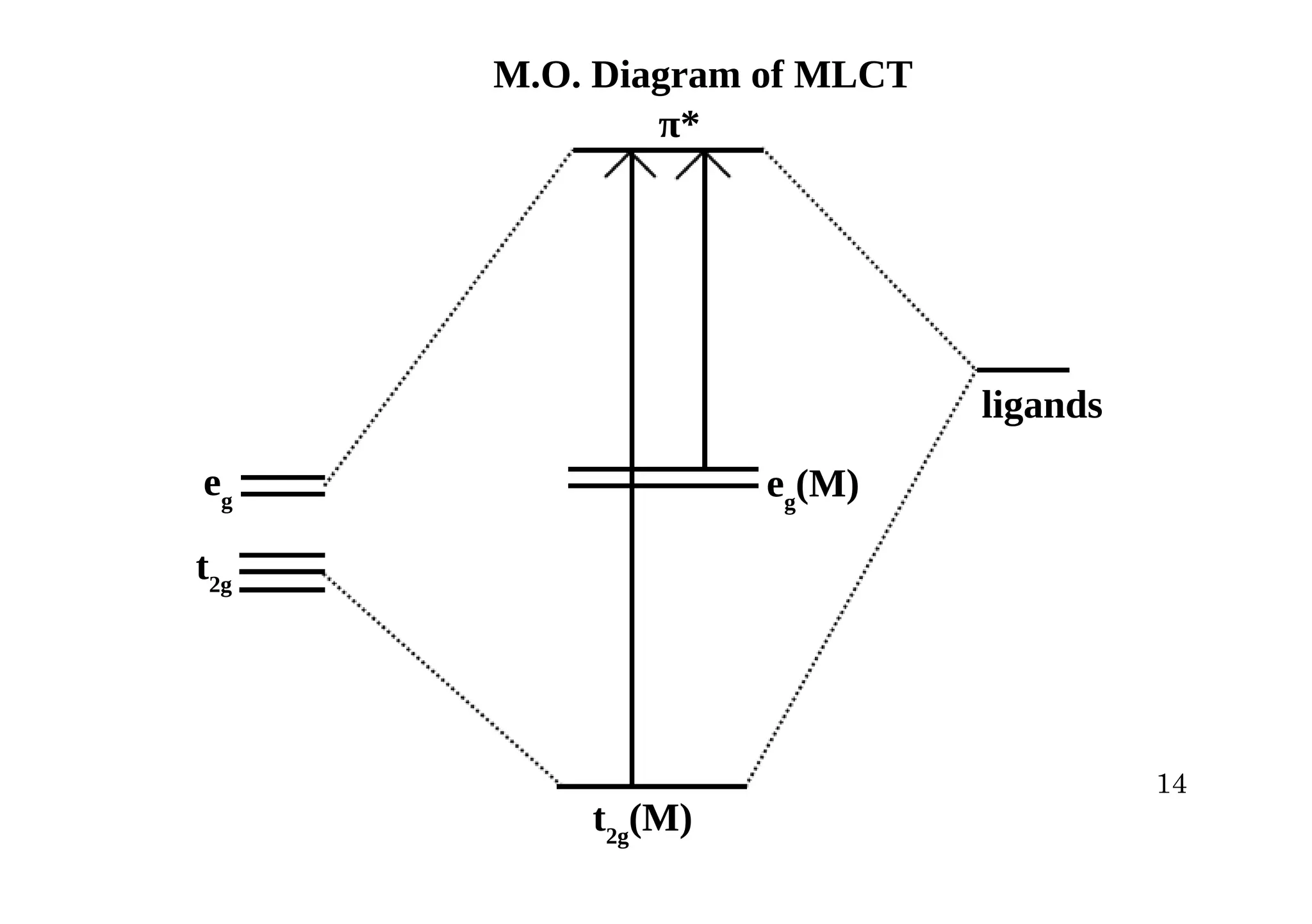
![Value of MLCT energy (energy difference between filled metal orbital and vacant ligand
orbital) depends on nature of metal orbital
(a) Electron rich metals, which have filled eg orbitals show coloured complex i. e. it absorbs
from visible region.
(b) If metals have filled t2g orbitals then MLCT energy is more, and complex absorbs from
uv region and complex will be colourless.
Metal to metal charge transfer (MMCT)
If in a complex same metal ions are present in different oxidation state, then electron
transfers between both metal ions is called MMCT.
Some examples are here---
(a) KFeIII
[FeII
(CN)6] Prussian Blue
KFeII
[FeIII
(CN)6] Turnbull’s Blue
(b) Rust ( Fe3O4 ) is reddish brown here elctrons transfers from Fe2+
-----→ Fe3+
(c) If spinels (mixed oxides) are coloured, it is due to MMCT eg. Pb3O4
15](https://image.slidesharecdn.com/charge-transfer-spectra-240214214416-90cea679/75/Charge-Transfer-Spectra-metal-to-metal-metal-to-ligand-15-2048.jpg)

