Embed presentation
Download to read offline

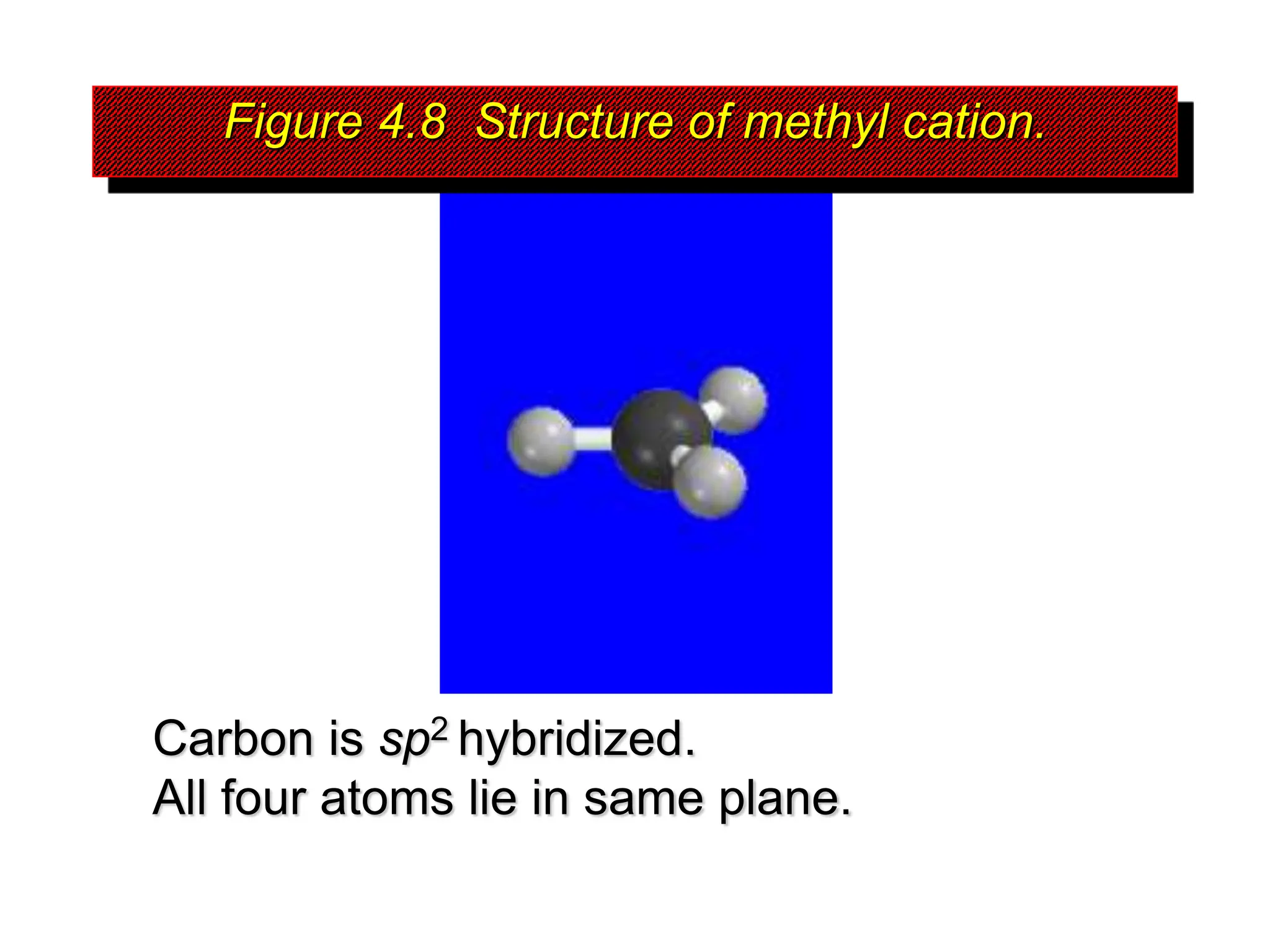



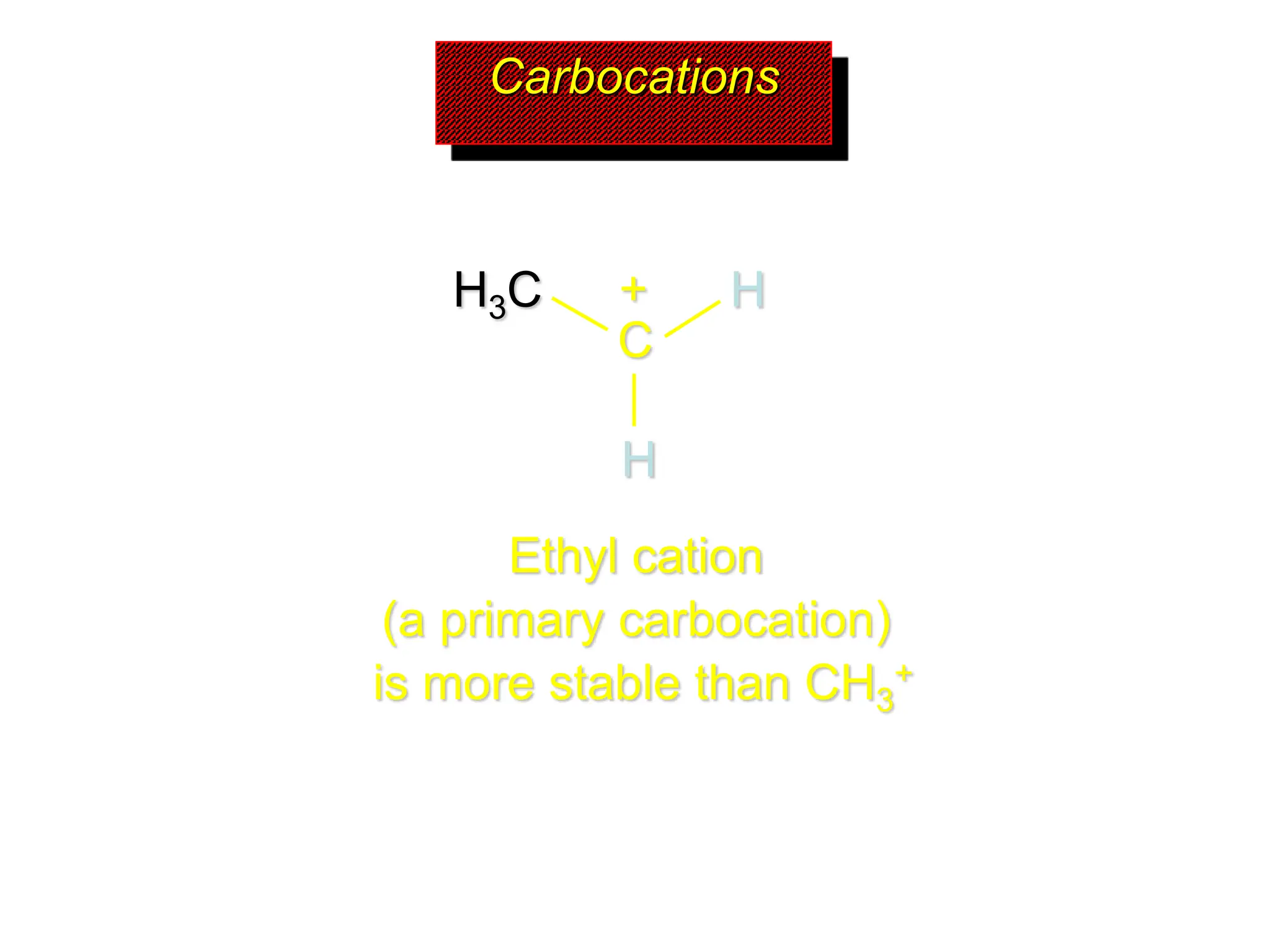
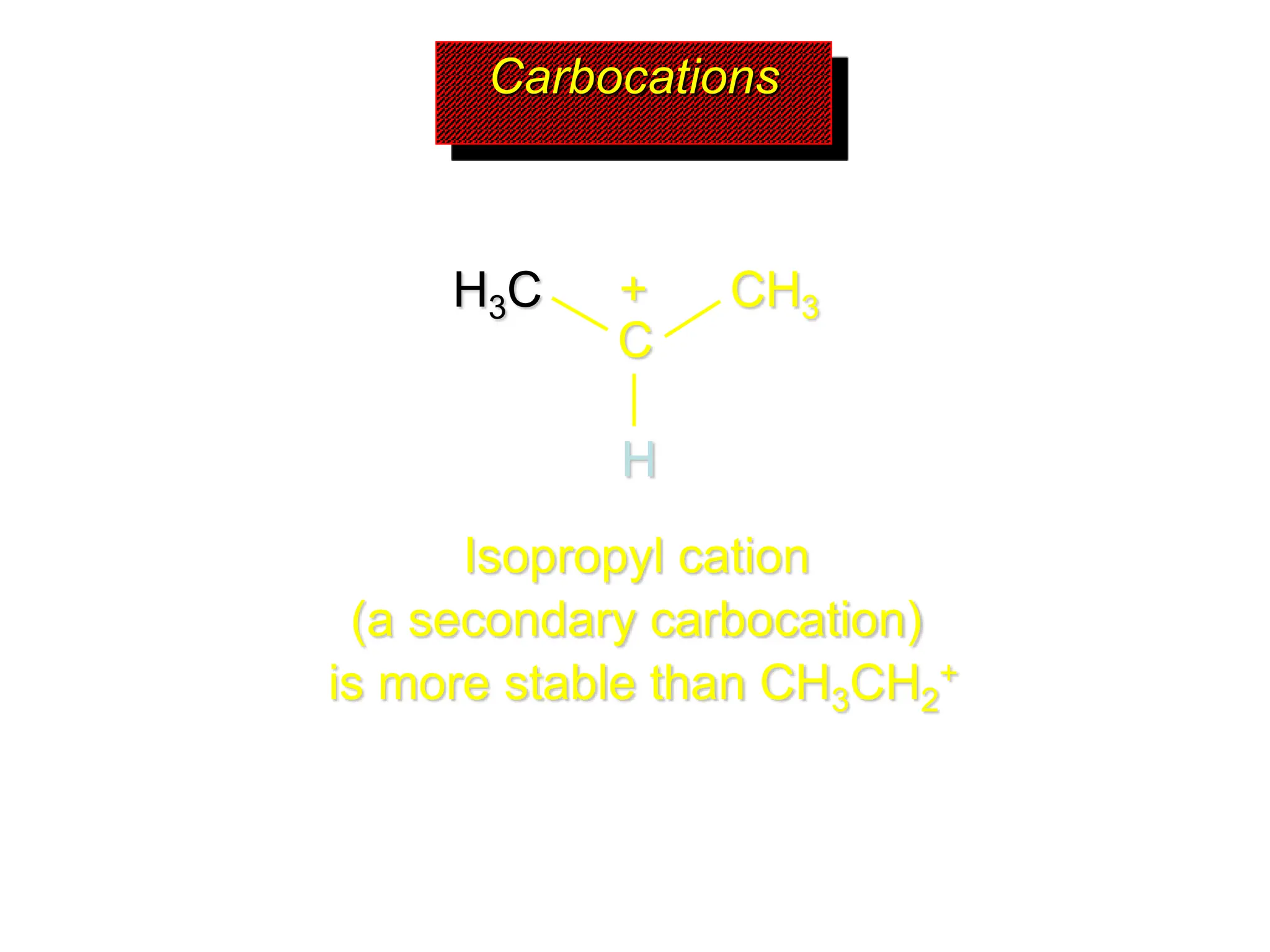

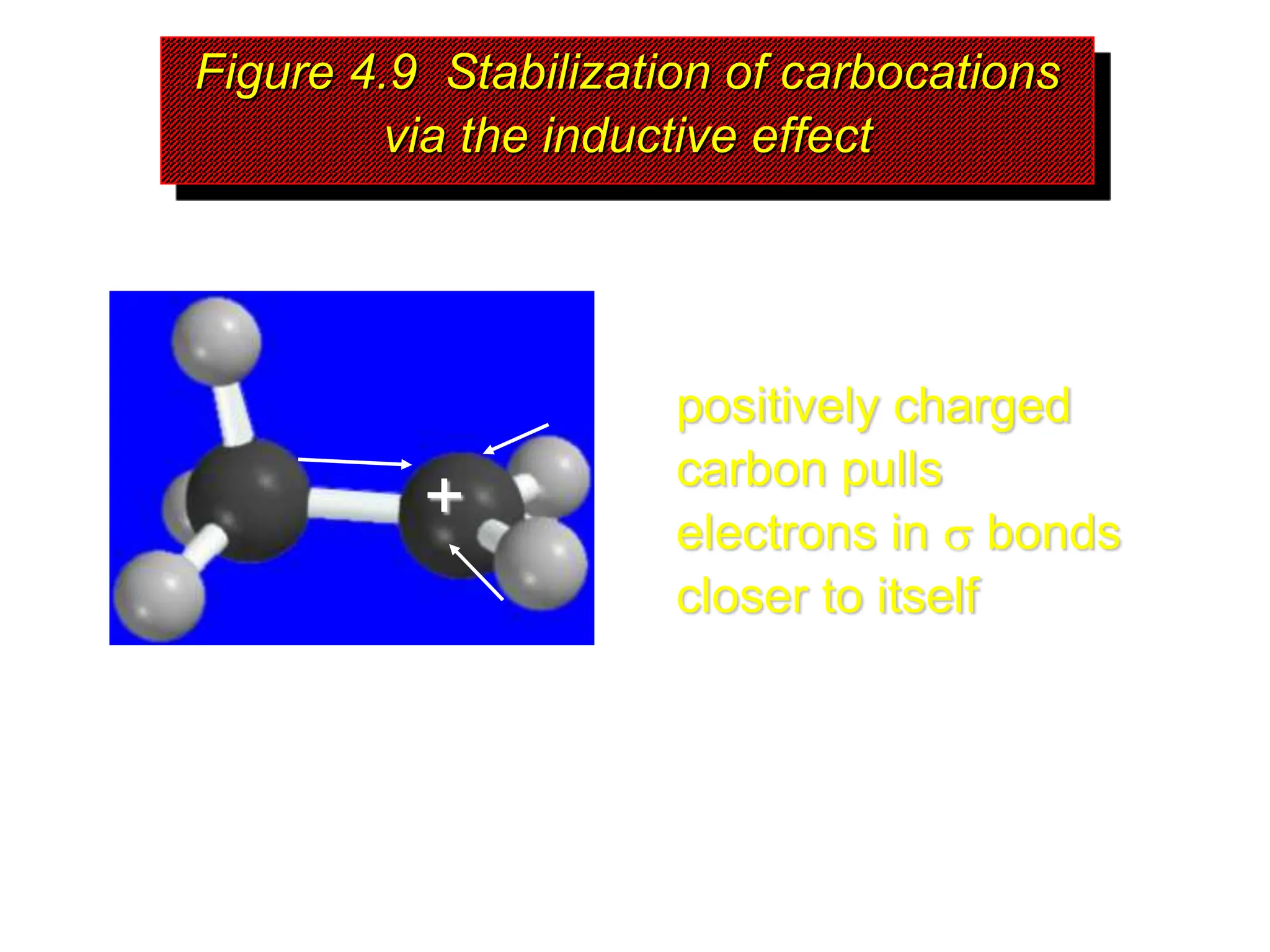

The document discusses the structure, bonding, and stability of carbocations, particularly highlighting the methyl cation's sp2 hybridized structure and empty 2p orbital. It explains that most carbocations are unstable, but their stability increases with alkyl group substitutions, with tertiary carbocations being the most stable. Additionally, it describes how the inductive effect helps in stabilizing positively charged carbons by dispersing the positive charge among adjacent atoms.









