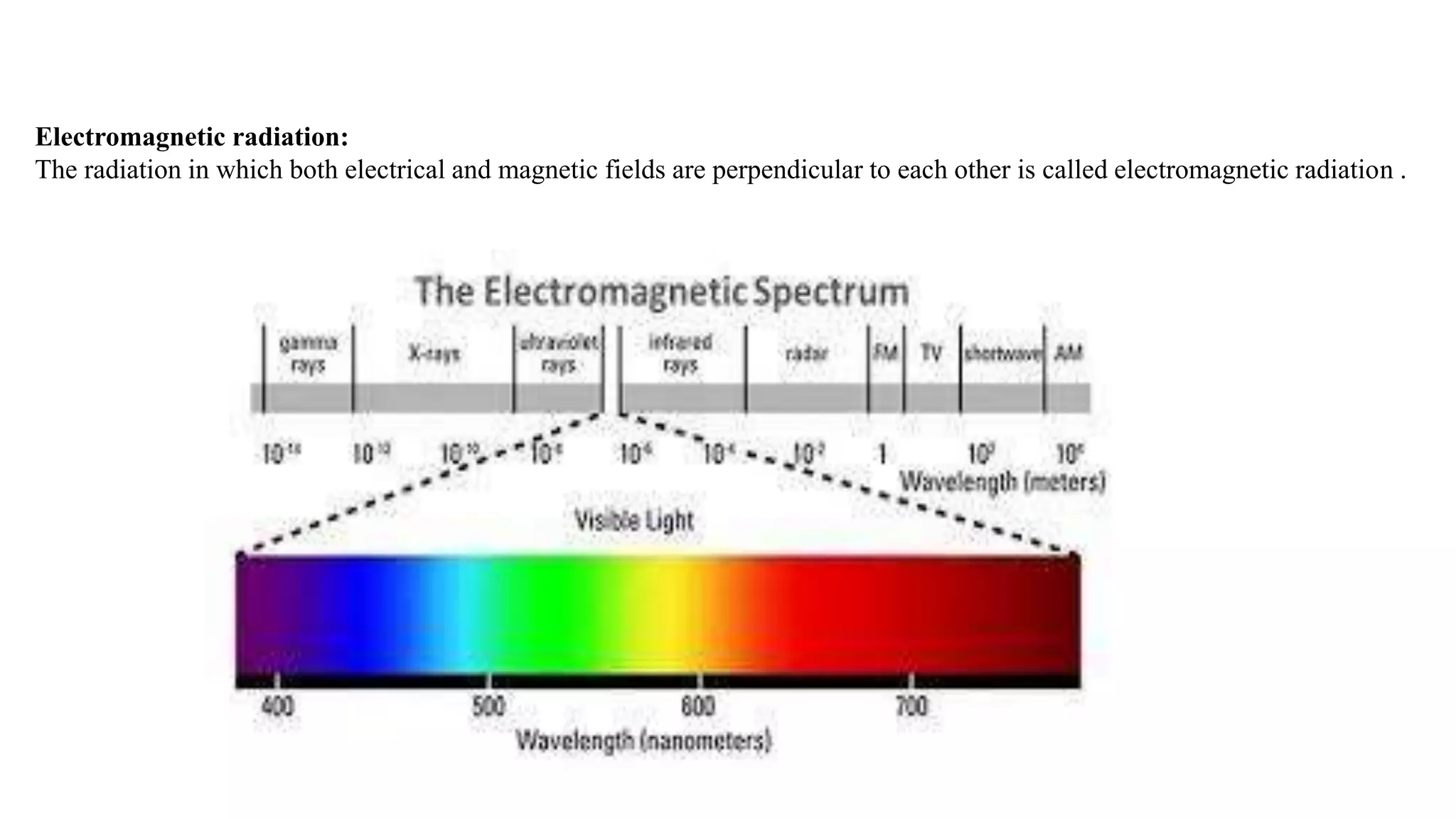
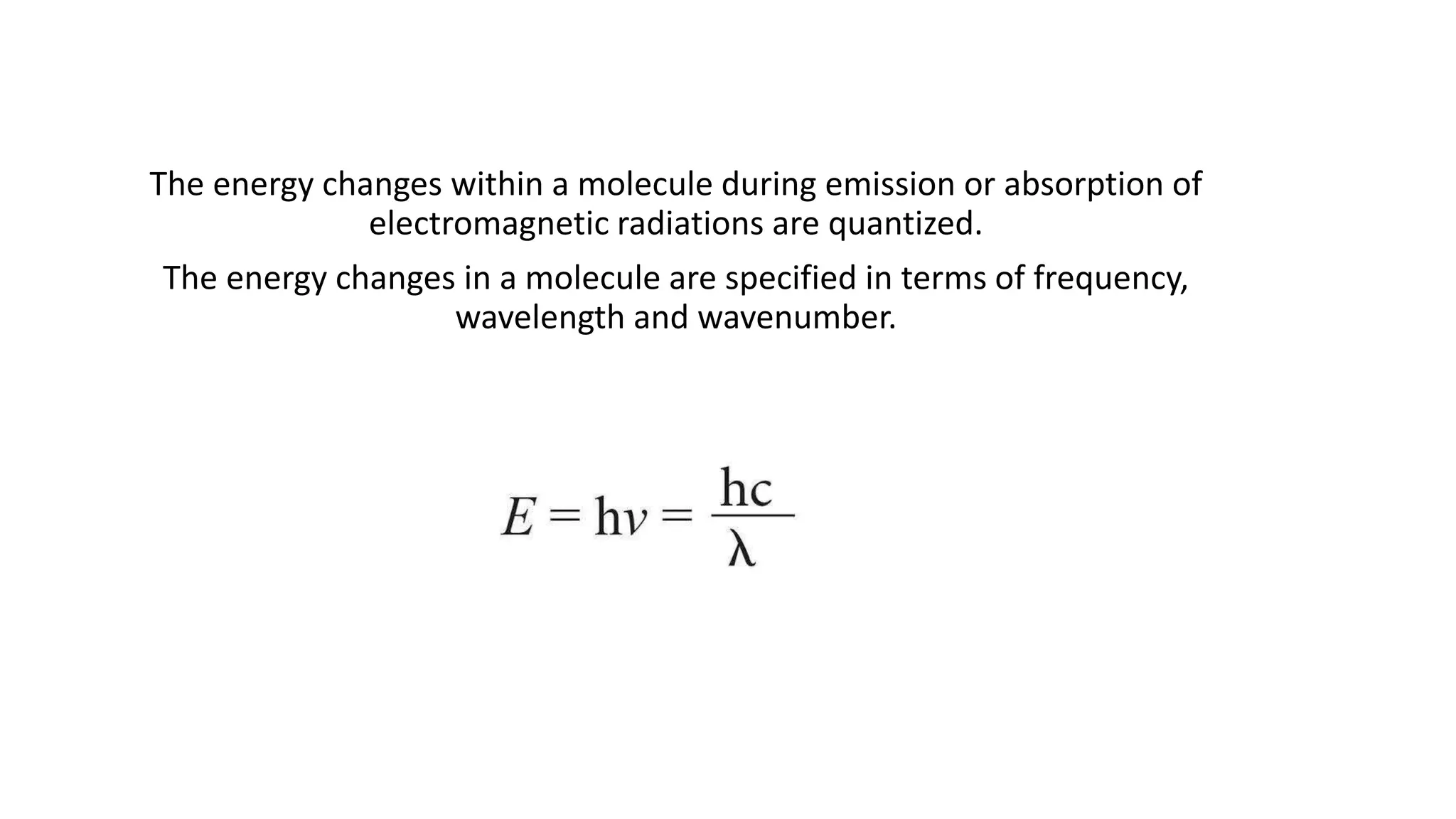
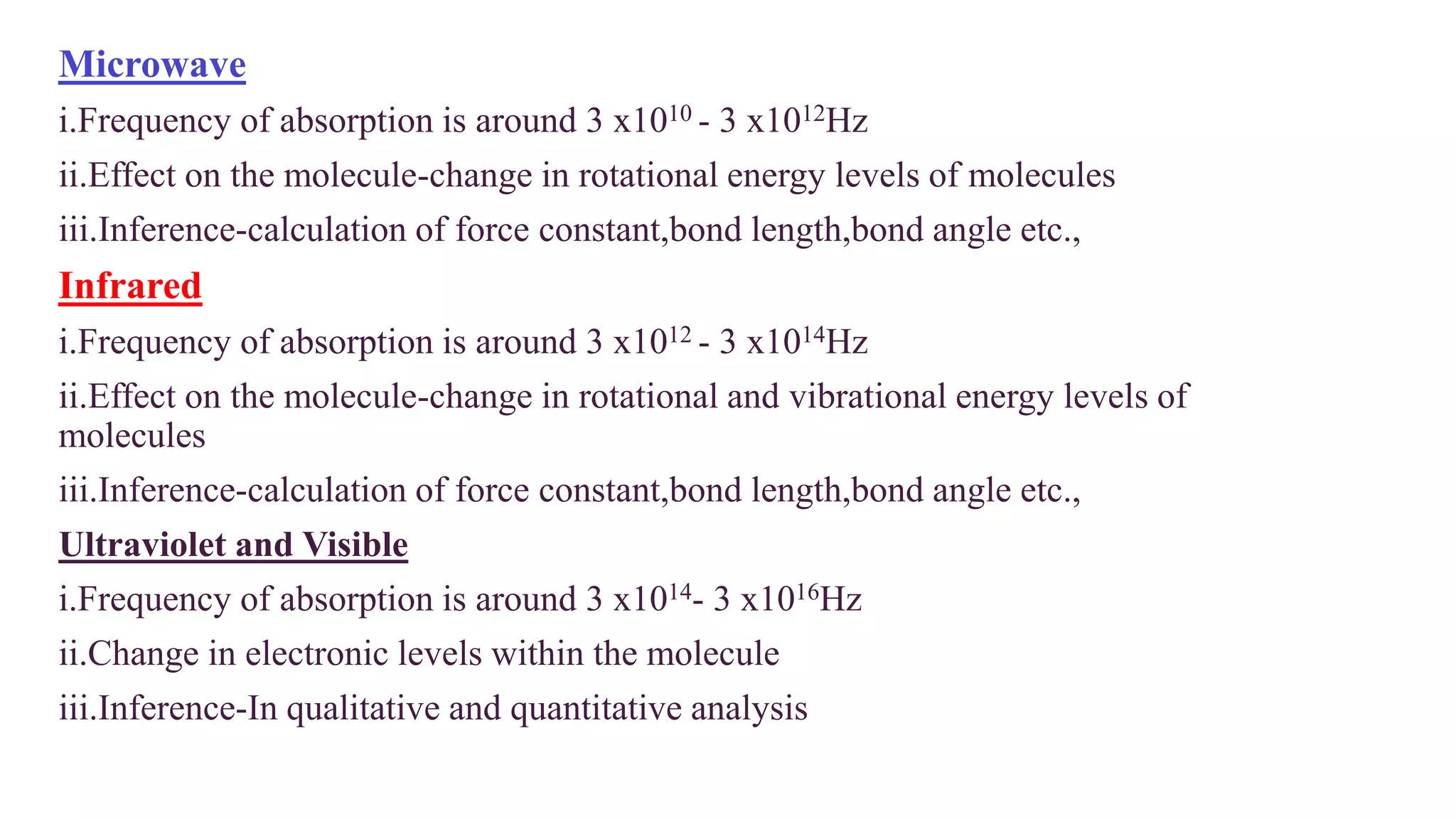
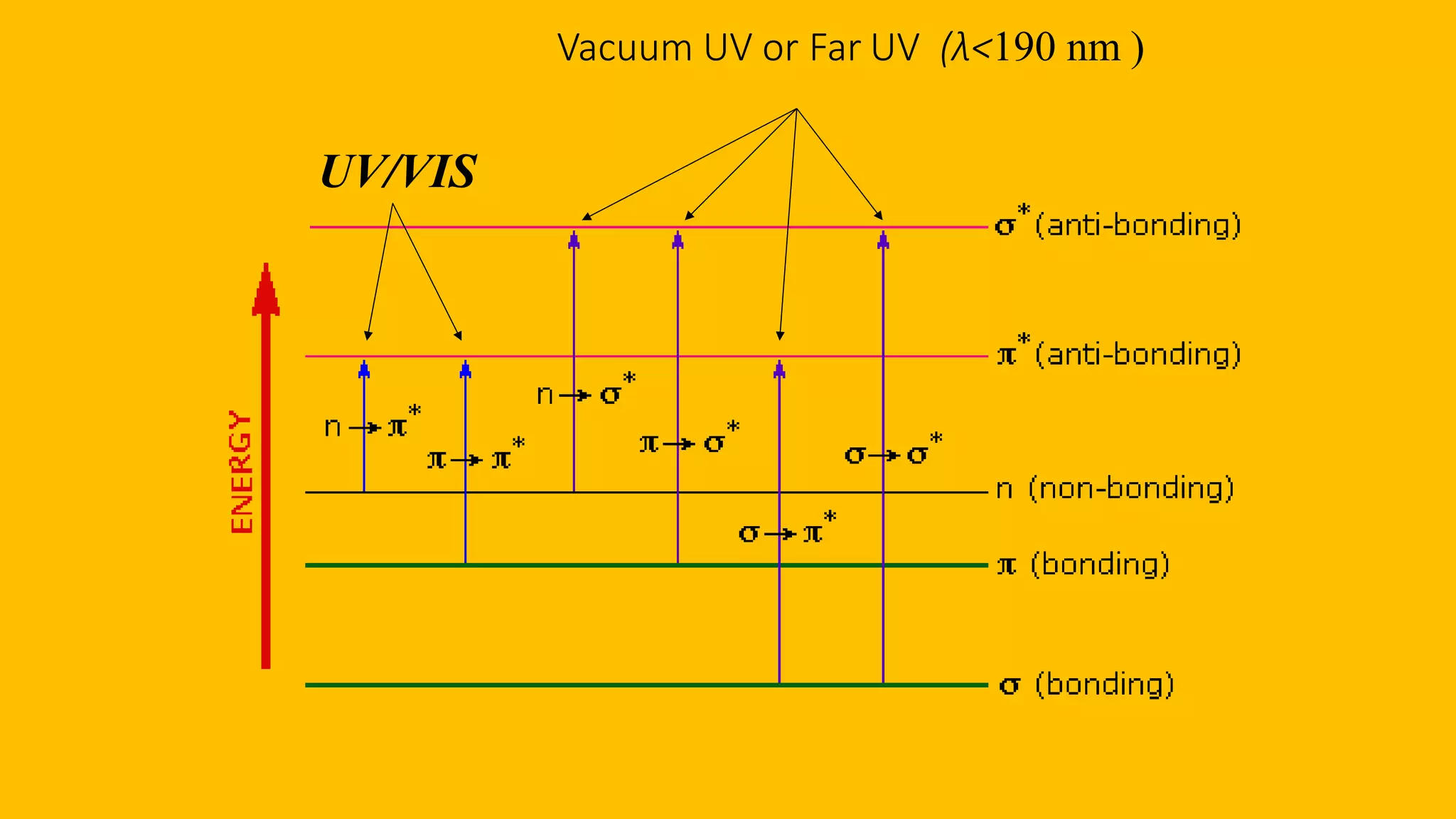
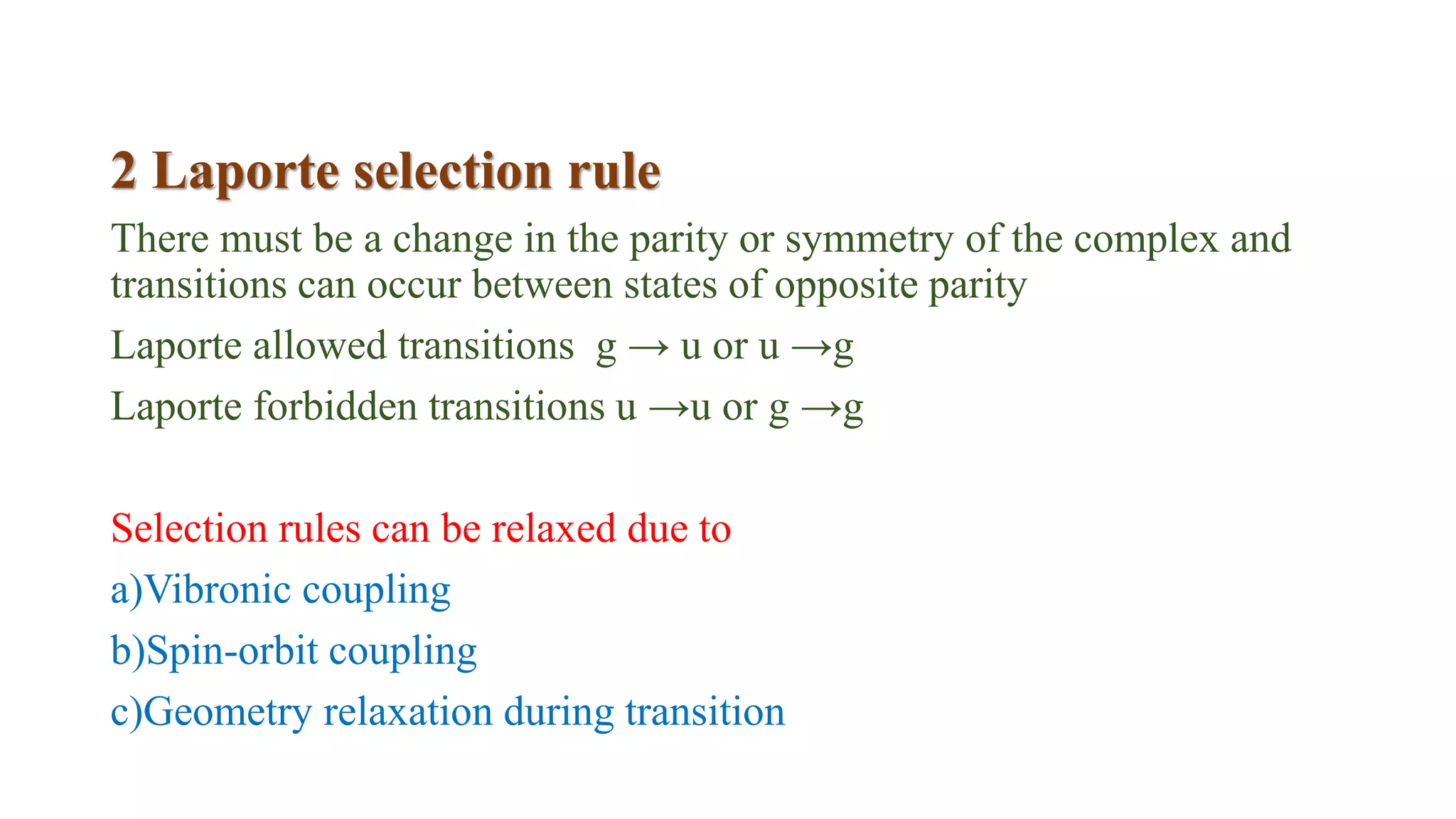
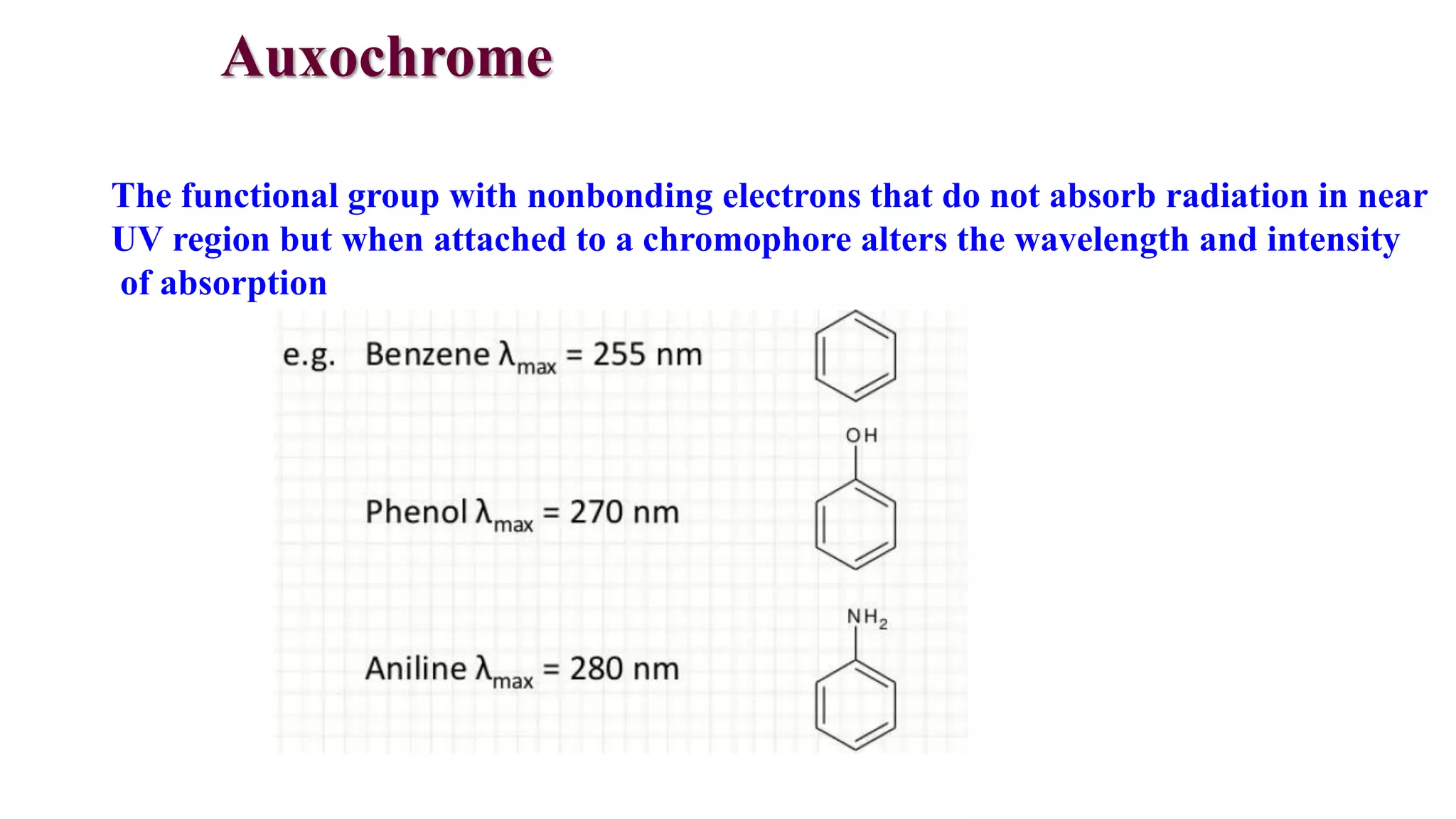
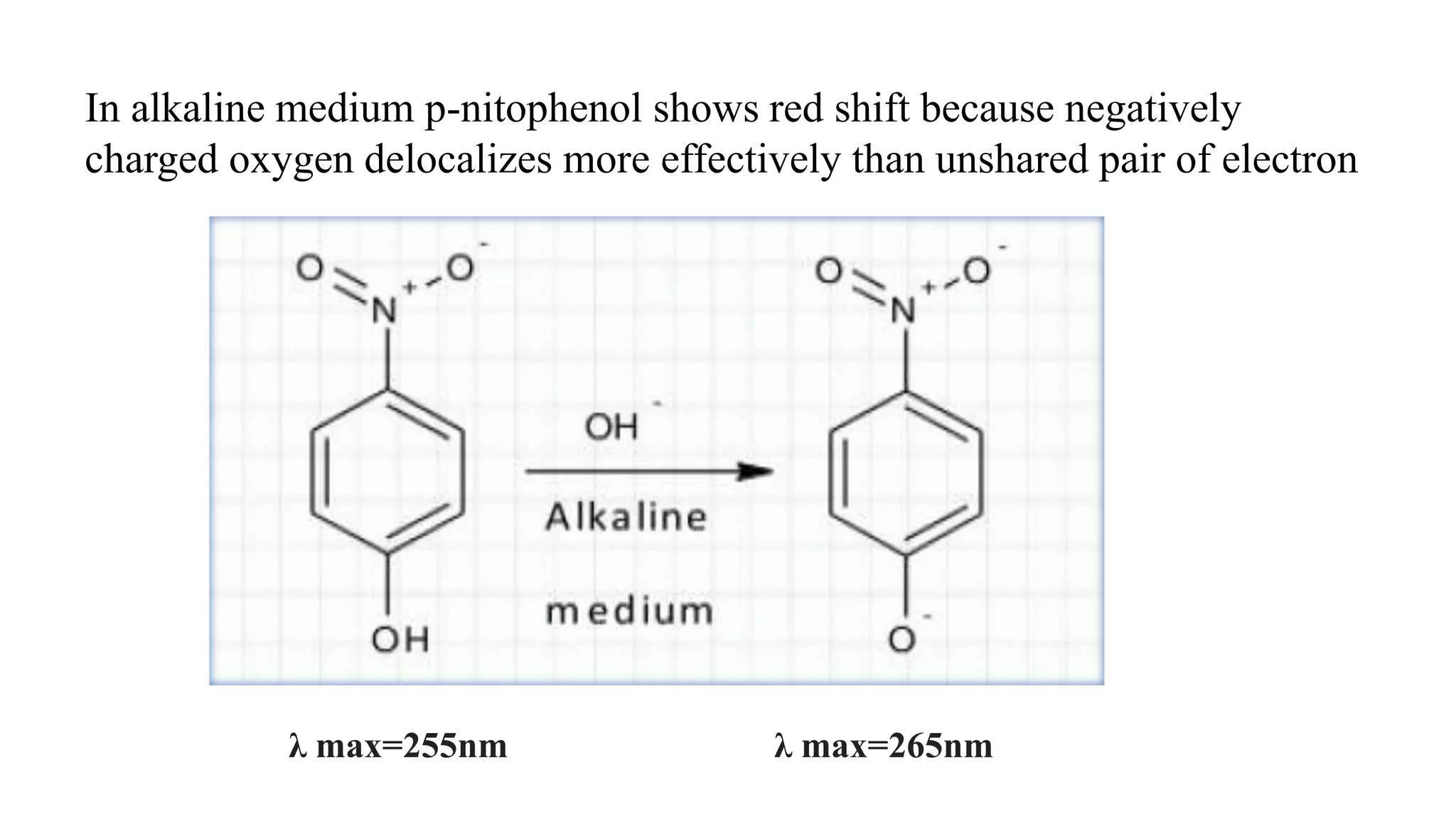
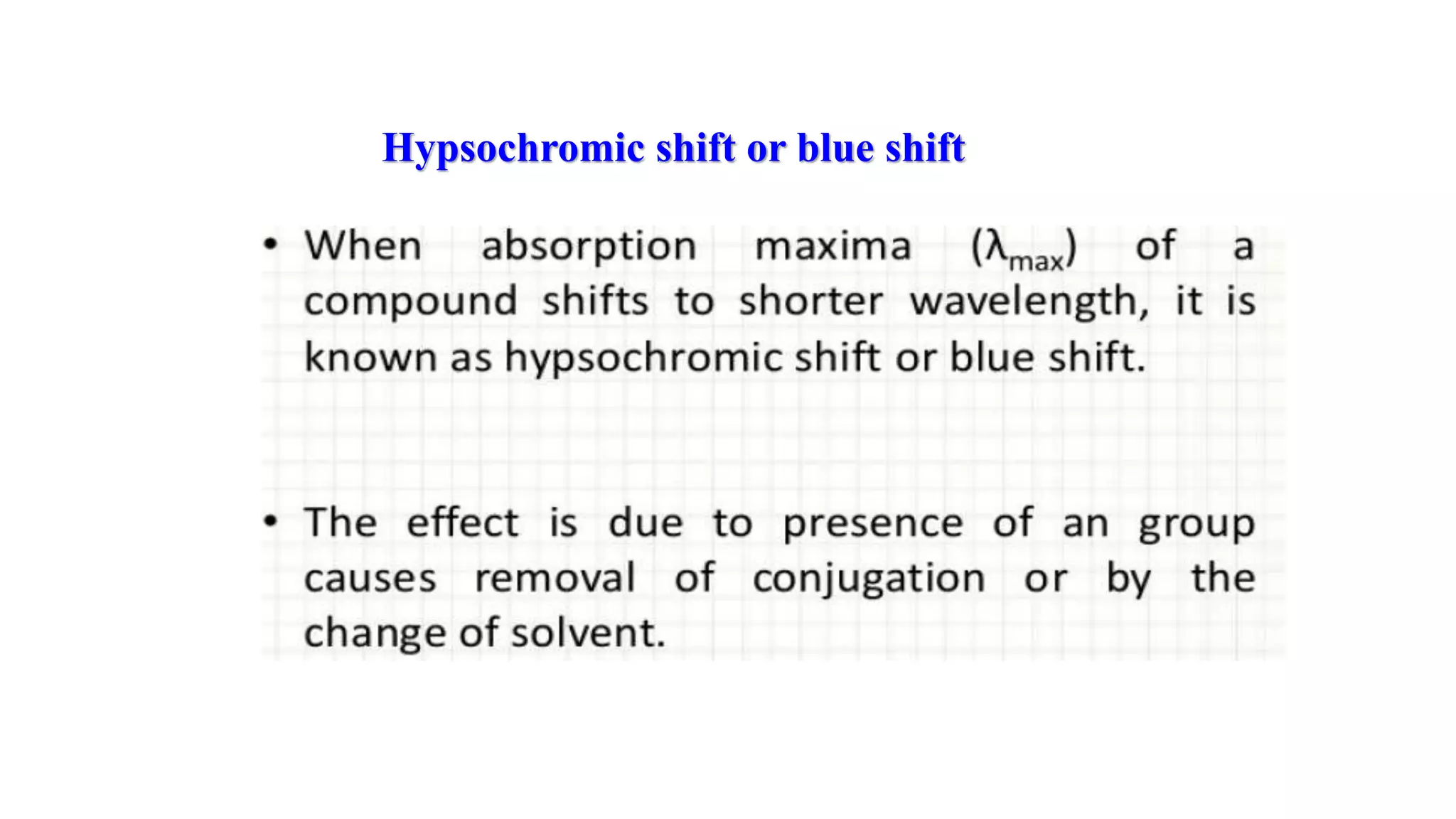
This document discusses UV spectroscopy and summarizes key concepts. It defines spectroscopy as the study of interaction between electromagnetic radiation and molecules. UV spectroscopy specifically examines electronic transitions that occur when molecules absorb ultraviolet or visible light. These transitions typically involve excitation of π or non-bonding electrons. The document outlines allowed and forbidden electronic transitions based on selection rules, and describes how auxiliary groups can shift the wavelength and intensity of light absorbed.