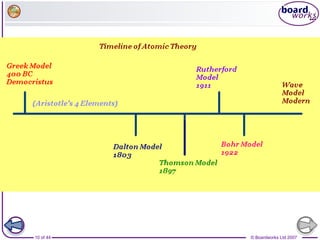
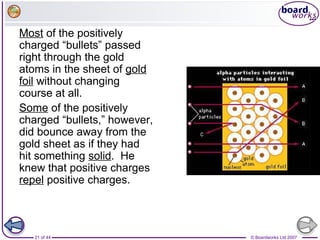
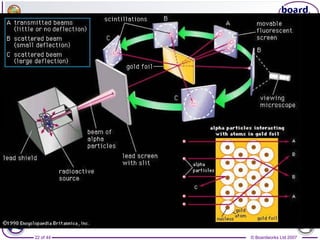
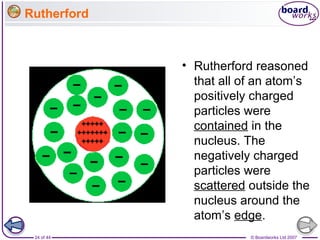
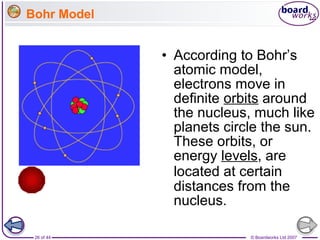
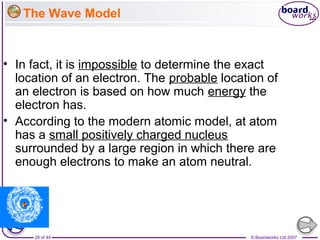
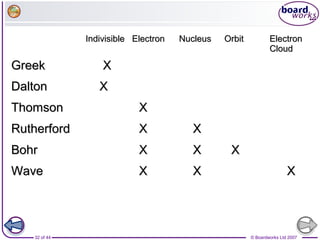
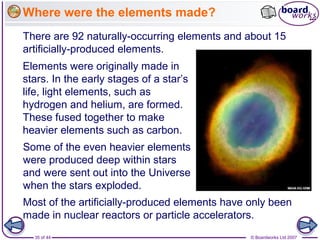
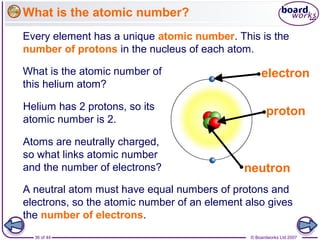
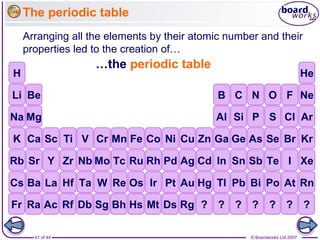
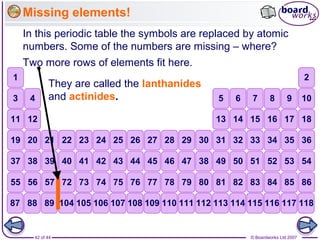
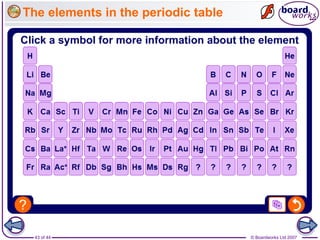
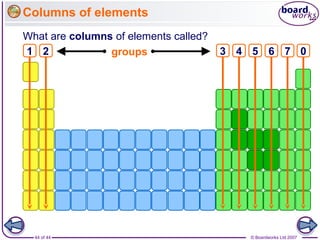
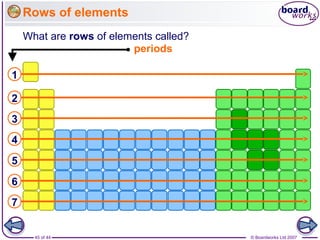
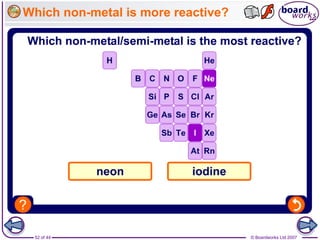
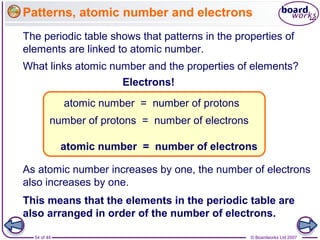
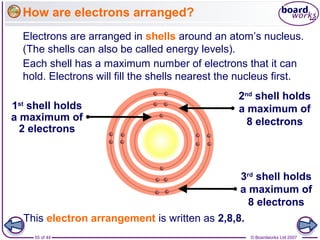
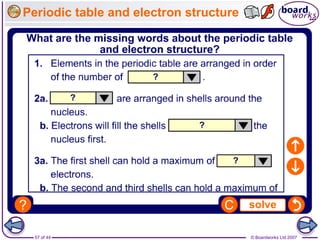
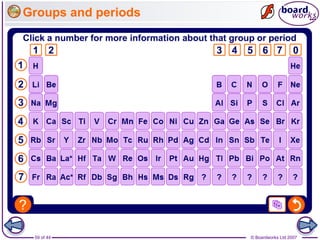
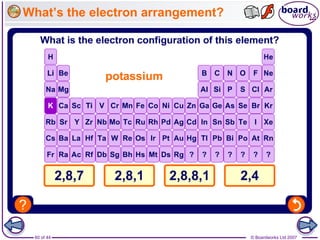
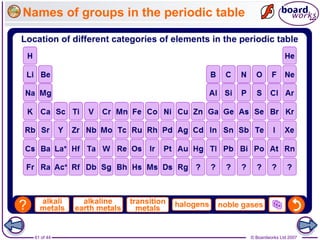
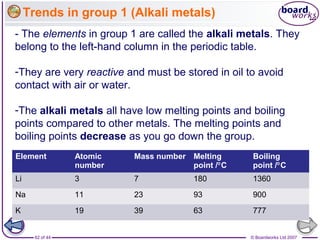
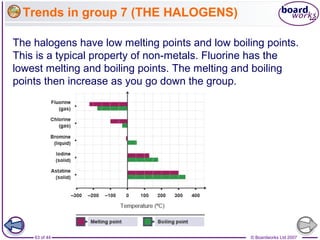
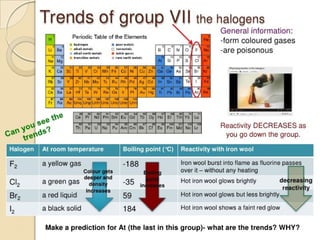
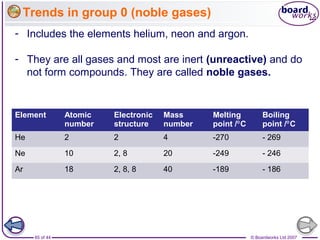
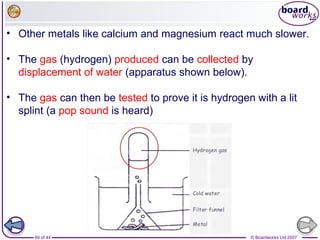
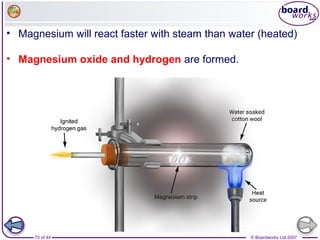
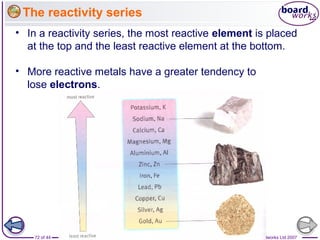
The document outlines the historical development of atomic models from Democritus' indivisible 'atomos' concept to modern atomic theory. Key figures such as John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr contributed foundational ideas about atomic structure, culminating in the wave model that describes electrons in probabilistic terms. Additionally, it discusses the organization of elements in the periodic table, their atomic numbers, and properties, along with trends observed in different groups of elements.