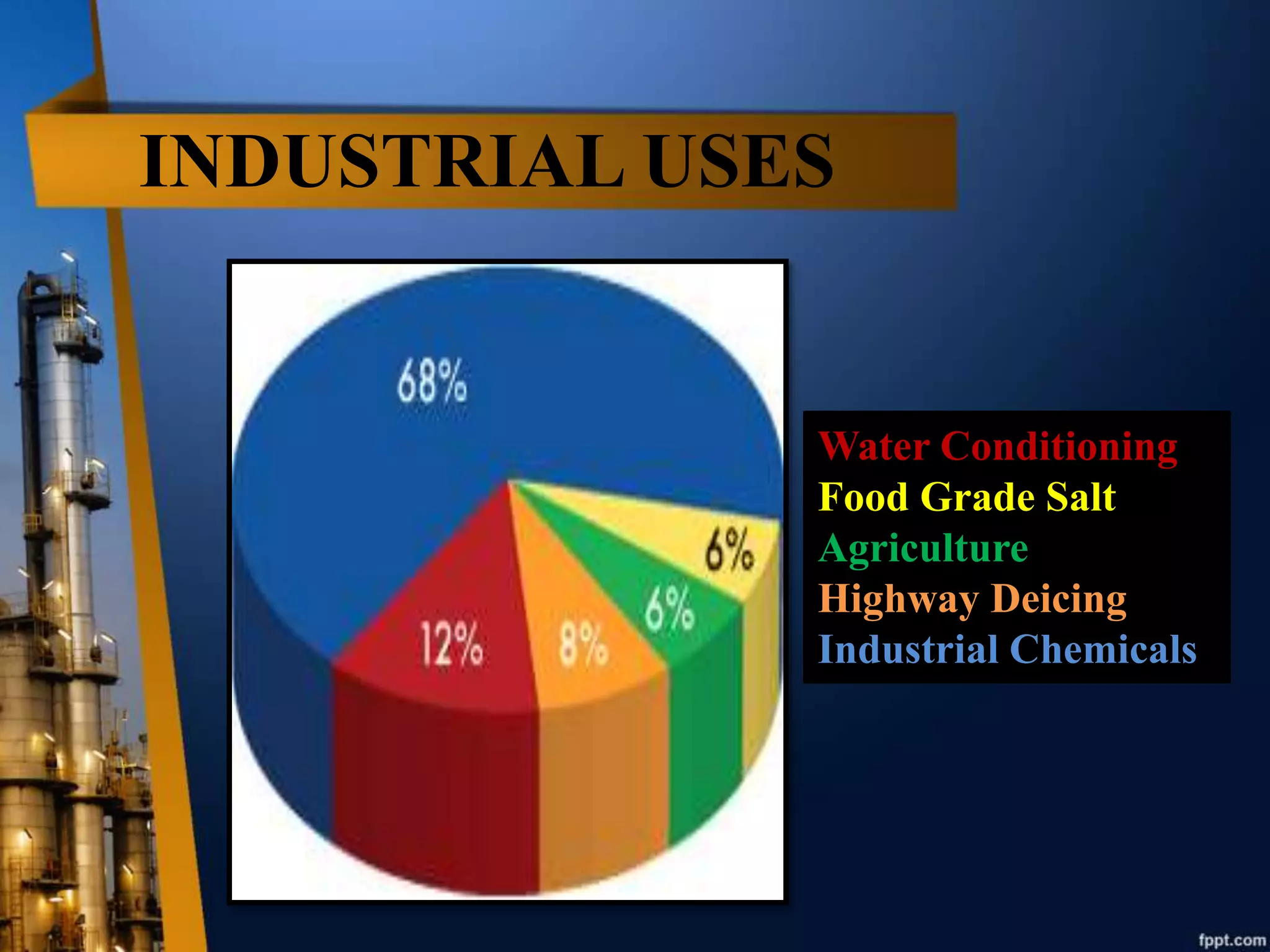
Salts are ionic compounds that result from the neutralization reaction of an acid and base. Salts exist in many colors and can be transparent, opaque, metallic, or lustrous. They are used for various industrial purposes like water conditioning, deicing, and producing chemicals. Salts can be produced through deep shaft mining, solution mining, or solar evaporation. Deep shaft mining involves drilling, blasting, crushing, and transporting salt rock, while solution mining dissolves salt beds with injected water that is then pumped out and evaporated.