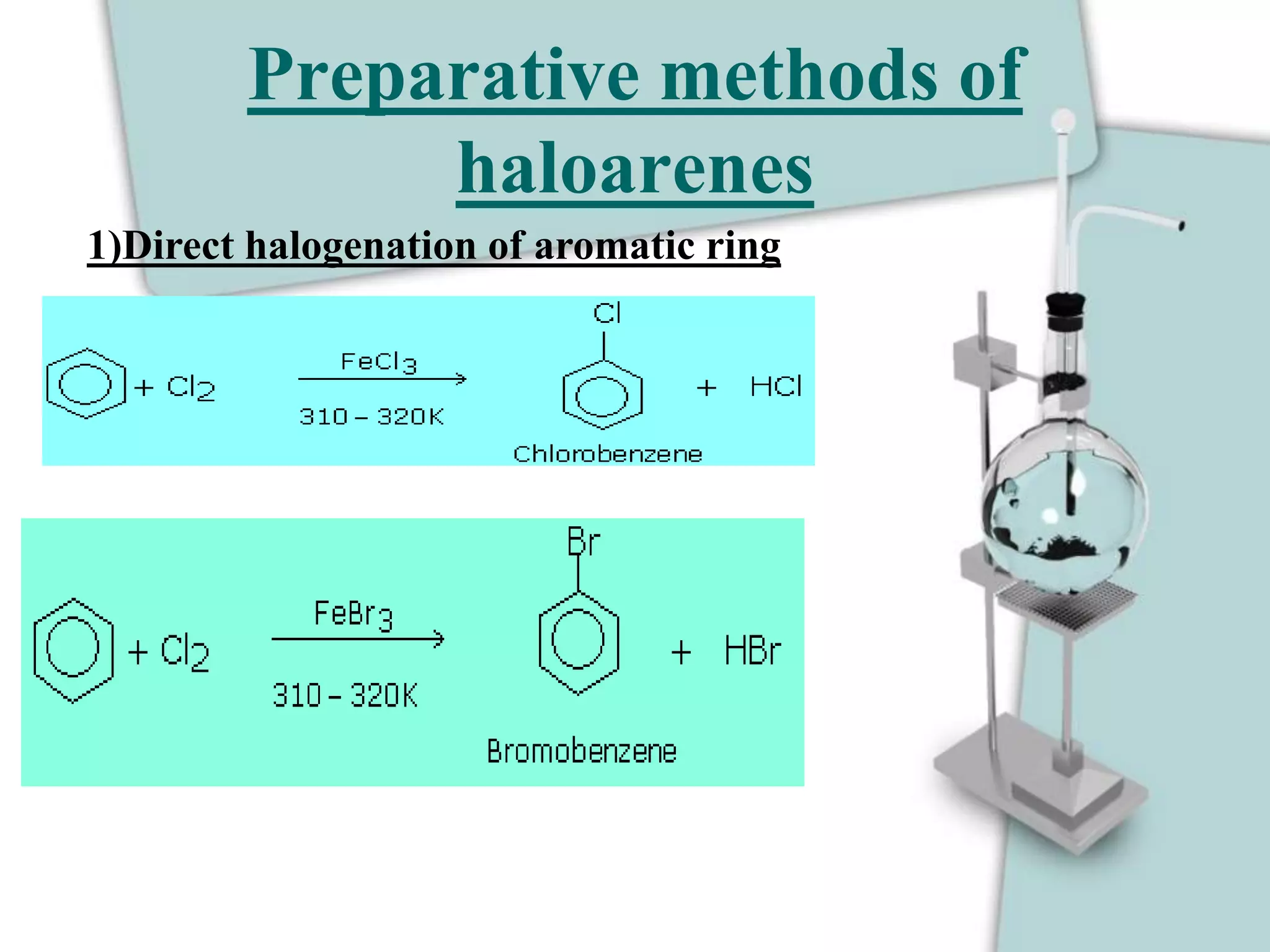
The document provides guidelines for units 10-13 of a syllabus covering nomenclature, reasoning, name reactions, reaction mechanisms, and word problems involving properties and reactions of functional groups. It discusses the naming and nature of alkyl halides, haloarenes, and their methods of preparation including from hydrocarbons, alkenes, alcohols, thionyl chloride, halide exchange, silver salts, and direct halogenation of aromatic rings. Bond dissociation energy decreases with increasing halogen size as C-Cl > C-Br > C-I.