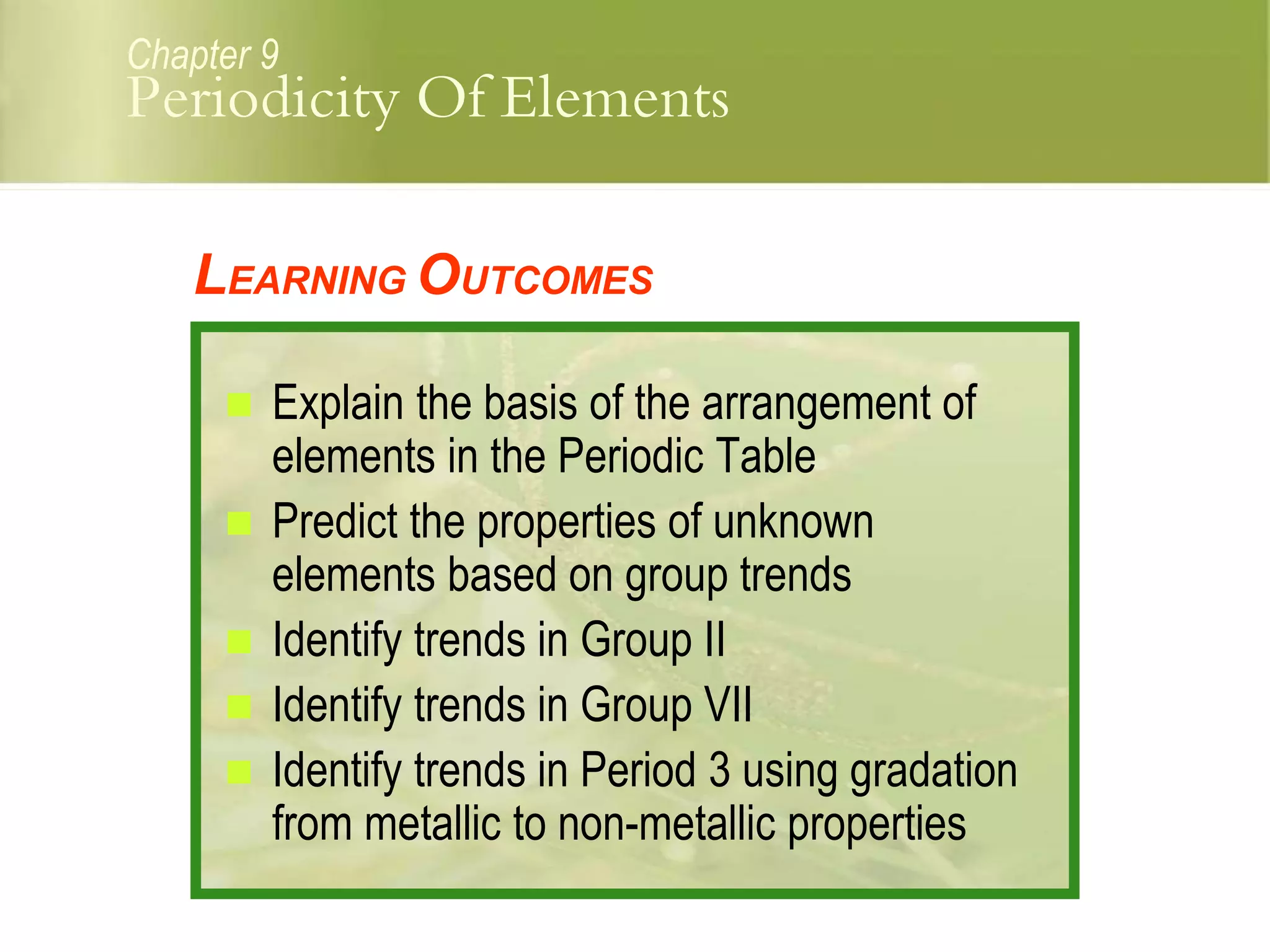
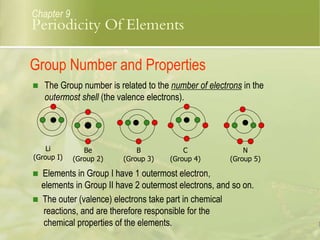
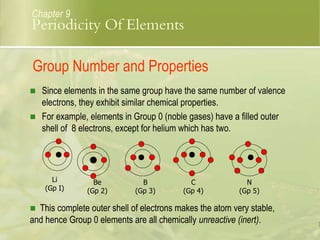
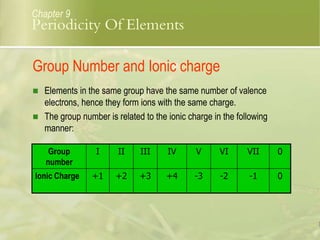
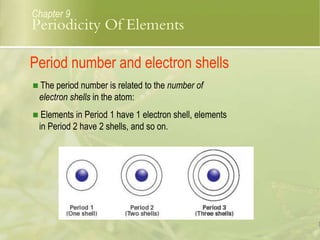
This document discusses periodic trends in elements across periods and down groups in the periodic table. It explains that elements in the same group have the same number of valence electrons and thus similar chemical properties, while periods are related to the number of electron shells. Metals are on the left side of the table and become more nonmetallic from left to right across periods as atomic number increases.