qdoc.tips_chemistry-form-4-chapter-4.pdf
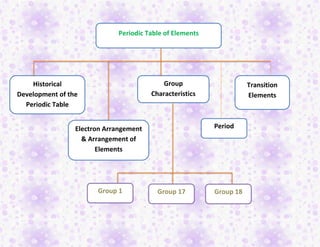
- 1. Periodic Table of Elements Historical Development of the Periodic Table Electron Arrangement & Arrangement of Elements Group Characteristics Period Transition Elements Group 1 Group 17 Group 18
- 2. Scientists Discoveries 1 Atoinne Lavoiser (1743 – 1794) -In 1789, first Chemist try to classify the substance, include the light & heat into metals & non-metals. -Unsuccessful bcoz light, heat & a few other compounds were also considered as elements 2 Johann W Dobereiner (1780 – 1849) -In1829,he divided the elements into the groups of 3 elements with similar chemical properties -Known as Dobereiner’s Traid -The atomic mass of the middle element was approximately the average atomic mass of the other 2 elements in each traid. -Traid system was confined to some elements only. -Led chemists to realise that there was a relationship between the chemical properties & the atomic mass of each element. 3 John Newlands ( 1837-1898) -From 1864 – 1865, he arranged the elements in order of increasing atomic mass. -Elements with similar properties recurred at every 8 element.
- 3. -known as the Law of Octaves. -Failure bcoz the Law of Octaves was obeyed by the 17 first elements only. -Shows the existence of a periodic pattern for the properties of elements. 4 Lothar Meyer (1830-1895) -In 1870 , he plotted graph of the atomic volume against the atomic mass. -Realised that elements with similar chemical properties occupied equivalent positions on the curve. -He found that the properties of the elements formed a periodic pattern against their atomic masses. 5 Dmitri Mendeleev (1834-1907) -In 1869, he arranged the elements in order of increasing atomic mass & grouped them according to similar chemical properties in vertical coloum. -He left gaps (empty spaces) in the table to be filled by undiscovered elements.
- 4. -He was able to predict the properties of undiscovered elements. 6 Hendry J.G. Moseley (1887- 1915) -In 1914,he studied the X-ray spectrum of elements. -From experiment, he concluded that proton number should be the basic for the periodic change of chemical properties instead of the atomic mass. -He arranges the elements in order of increasing proton number in the Periodic Table. Thus, he confirmed the works / Mendeleev. -The modern Periodic Table based on the foundation of Henry J.G Moseley.
- 5. 1) Arranged according to ascending proton number of the element. 2) 1-18 called Group. 1-7 called periods
- 6. Group 1) Group arranged according to the number of valence electron in the outermost shells. 2) All elements in a group a) Have the same valence electrons b) Have the same chemical properties c) Physical properties will change as we go down the group. 3) Groups have special names. Group Name 1 Alkali metals 2 Alkaline earth metals 17 Halogens 18 Inert (noble) gases 3-12 Transition metals 4) Group 1,2,13 & transition elements are metals. Group 15-18 are non-metals. Group 14 has 2 non-metals ( carbon& silicon)
- 7. Period 1) Period arranged according to the number of shells occupied with electron in an atom. 2) Elements in period 1 – 7 Period Number of elements Period 1 2 elements Period 2 & 3 8 elements Period 4 & 5 18 elements Period 6 32 elements Period 7 23 lements
- 8. Electrons Arrangement 1) Number of filled electron shells increases, as we go down the group. 2) By knowing the proton number of the element, we can determine the group & period it’s placed in. 3) Relationship between number of valence electrons with the group number of an element. No.of valence electrons 1 2 3 4 5 6 7 8 (second 8. First 8 is in transition elements except helium) Group 1 2 3 14 15 16 17 18 9) Number of electrons = number of protons 10) The number of shells = period 11) Valence electron = group
- 9. 12) Maximum number of electron which occupy each shell Shell number Maximum number of electrons 1 2 2 8 3 8/18 4 46 Exp: Electron arrangement =2.4 Carbon Valence electron 4 so it is placed in group 14 First shell has 2 electrons & second shell has 4 electrons. So it has 2 shells & it is in period 2. Shell Electron
- 10. Element Proton number Electron arrangement Number of electron valence Group Number of shell Period H 1 1 1 1 1 1 He 2 2 2 (duplet) 18 1 1 Li 3 2.1 1 1 2 2 Be 4 2.2 2 2 2 2 B 5 2.3 3 13 2 2 C 6 2.4 4 14 2 2 N 7 2.5 5 15 2 2 O 8 2.6 6 16 2 2 F 9 2.7 7 17 2 2 Ne 10 2.8 8(octet) 18 2 2 Na 11 2.8.1 1 1 3 3 Mg 12 2.8.2 2 2 3 3 Al 13 2.8.3 3 13 3 3
- 11. Group 18 - Helium(He),Neon(Ne),Argon(Ar),Krypton(Kr),Xenon(Xe) & Radon(Rn) - Noble/inert gases that exists in monoatomic gases. Physical properties 1) Can’t dissolve in water , can’t conduct electricity & heat 2) Low densities 3) Colorless gaseous state at room temperature & pressure 4) Melting & boiling point is low.
- 12. 5) Changes of physical properties when going down group 18: Properties Changes Explanation Atomic size increases Bcoz the number of occupied shells increases. Melting & boiling point increase Bcoz 1)the atomic size increaseswhen going down the group 2)The force of attraction between particles become stronger 3)Thus, more heat is needed to overcome the stronger force Density increase because the mass increases
- 13. Chemical Properties 1) Unreactive/inert 2) Atoms don’t need to donate, accept/share electrons bcoz the electron arrangement of the noble gas atoms are stable. Uses Elements Uses Helium -Fill air ships/weather balloons -Gas in diving tank Neon -Light up advertising boards/lights Argon -Fill in filament bulbs Krypton -Used in flash bulbs & lasers Randon -For cancer treatments Xenon -Used in flash bulbs & lasers
- 14. Group 1 - Lithium (Li) , Sodium (Na) , Potassium (K) , Rubidium (Rb) , Caesium (Cs) & Francium (Fr) - Known as alkali metals. Physical properties 1) Conduct heat & electricity 2) High melting point & boiling point 3) All alkali metals are grey in colour with shiny surfaces but they are soft, can cut easily
- 15. 4) Changes in physical properties going down the group: Properties Changes Explanation Atomic size Increases Because the number of occupied shells increases Melting & boiling point Decreases Because 1)The atomic size increases when going down the group. 2)The metal bond between atoms become weaker 3)Thus,less heat is needed to overcome the weaker bond. Density increases Because increase in atomic size so it became heavier. Chemical Properties 1) Very reactive elements. 2) The reactivity increases when going down the group. The valence electron in the outermost shell become further from the nucleus. The forces of attraction of nucleus on the valence electron become weaker. The valence electron becomes easier to release.
- 16. Chemical properties Chemical equations Reacts with water to produce alkaline metal, hydroxide solutions & hydrogen gas 2 X + 2 H2O 2 XOH + H2O Burns in oxygen gas to produce white, solid metal oxides 4 X + O2 2 X2O Burns in chlorine gas to form white solid, metal chloride. 2 X + Cl2 2 XCl X= metals in group 1 (Li,Na,K,Rb,Cs,Fr) Safety Precautions when handling Alkali metals 1) Kept in paraffin oil 2) Use forceps to take the metal 3) Wear safety goggles & gloves
- 17. Group 17 1) Fluorine (F) , Chlorine (Cl) , Bromine (Br) , Iodine (I) & Astatine (At) 2) Known as halogens 3) Exists in Diatomic molecules, which F2,Cl2,Br2,I2,At2 4) Non-metals Physical properties 1) Doesn’t conduct heat & electricity bcoz they consist of covalent molecules. 2) Low melting point & boiling point bcoz their molecules r attracted to each other by intermolecular force. Force of attraction & intermolecular force are the same. Means the forces between 2 molecules
- 18. 3) Changes of properties when going down the group 17. Properties Changes Explanation Atomic size Increases Because the number of shells increases Melting & boiling point Increases Because 1)The atomic size increases when going down the group. 2)The force of attraction between particles become stronger. 3)Thus,more heat is needed to overcome the stronger force. Colour Darker Flourine = pale yellow gas Chlorine = Greenish-yellow gas Bromine = Reddish-brown liquid Iodine = purplish-black solid Density Increases Because increase in atomic size so it became heavier. Physical changes change from a gas (fluorine & chlorine) to liquid (bromine) to solid (iodine & astatine)
- 19. Chemical Properties 1) Same chemical properties bcoz all halogen atoms have 7 valence electrons but their physical properties are different. 2) Reactivity decreases down the group Chemical properties Chemical equations Reacts with water to produce 2 acids X2 + H2O HX + HOX Reacts with hot iron to form a brown solid iron halides 3 X2 + 2Fe 2FeX3 Reacts with sodium hydroxide solution,NaOH to form sodium halite(I) & water X2 + 2NaOH NaOX + H2O X = halogens (F,Cl,Br,I,At)
- 20. Elements across the period 1) Horizontal rows = periods 2) Consists of 7 periods which periods 1,2 & 3 are short periods. Others are long periods 3) The number of protons in the elements increases across the period from left to right. They increase by 1 proton Element Na Mg Al Si P S Ci Ar Proton number 11 12 13 14 15 16 17 18 Electron arrangement 2.8.1 2.8.2 2.8.3 2.8.4 2.8.5 2.8.6 2.8.7 2.8.8 Metallic properties Metals Semi metal Non-metals Physical properties Solid Electrical conductivity Good Average Poor Element oxide base amphoteric acid
- 21. Across Period 3 - Atomic size of elements increases - Electropositivity(accept) of elements increases - Electropositivity(easy to donate) of elements decreases - Electronegativity increases - Metallic properties decreases - Non-metallic properties increases Element Sodium Magnesium Aluminum Silicon Phosphorus Sulphur Chlorine Acid-base properties Base Base Amphoteric Acid Acid Acid Acid Reaction with acidic / alkaline solution Acid X Alkali Acid X Alkali Acid Alkali X Acid Alkali X Acid Alkali X Acid Alkali X Acid Alkali X = Insoluble = Soluble
- 22. Transition elements 1) Transitions elements have a) Shiny surface b) Ductile c) Malleable ( can easily shaped into different shapes) d) Hard e) High melting point & boiling point f) High density g) Conducts electricity & heat 2) a) Transition elements form coloured compounds & ions Element Ion Colour Chromium (Cr) Cr3+ CrO2 -4 Cr2O2-7 Green Yellow Orange Manganese (Mn) Mn2+ MnO- 4 Pale pink Purple Iron (Fe) Fe2+ Pale green
- 23. Fe3+ Yellowish brown Cobalt ( Co) Co2+ Pink Nickel (Ni) Ni2+ Green Copper (Cu) Cu2+ Blue( CuSO4) Green (CuCO3) b) Precious stones are coloured bcoz of the presence of these coloured ions in them Gemstone Transition metal Colour Emerald Amethyst Sapphire Ruby Topaz Ni & Fe Fe & Mn CO & Ti Cr Fe Green Purple Blue Red Yellow
- 24. c) They form complex ions which are coloured,such as: Hexacyanoferrate (II) ion , [Fe(CN)6]4- Hexacyanoferrate(II) ion , [Fe(CN)6]3- Hexaamina chromium(II) ion , [Cr(NH3)6]3+ Tetraamina copper(II) ion , [Cu(NH3)4]2+ Tetrachlorocuprate(II) ion , [CuCl4]2- d) All transition elements except scandium & zinc have more than 1 oxidation numbers. Compound Formula Oxidation number Chromium (III) chloride Potassium (VI) dichromate Manganese (II) sulphate Manganese (IV) oxide Potassium (VII) manganate Iron (II) sulphate Iron (III) chloride Copper (I) oxide Copper (II) sulphate CrCl3 K2Cr2O7 MnSO4 MnO2 KmNo4 FeSO4 FeCl3 Cu2O CuSO4 +3 +6 +2 +4 +7 +2 +3 +1 +2
- 25. e) Transition metals are good catalysts. A specific catalyst is used for a specific reaction,as in, Process Catalyst To manufacture Haber process Iron filings , Fe Ammonia Contact process Vanadium (V) oxide ( V2O5) Sulphuric acid Ostwald process Platinum , Pt Nitric acid Hydrogenation Nickel , Ni Margarine Uses of transition elements in industry 1) Mercury used in thermometer bcoz the melting & boiling point & liquid state are high 2) Tungsten used to make filament bulb as it doesn’t melt easily 3) Iron used to make vehicles, electrical appliances & other products 4) Chromium used to coat iron to prevent rusting of iron substances.