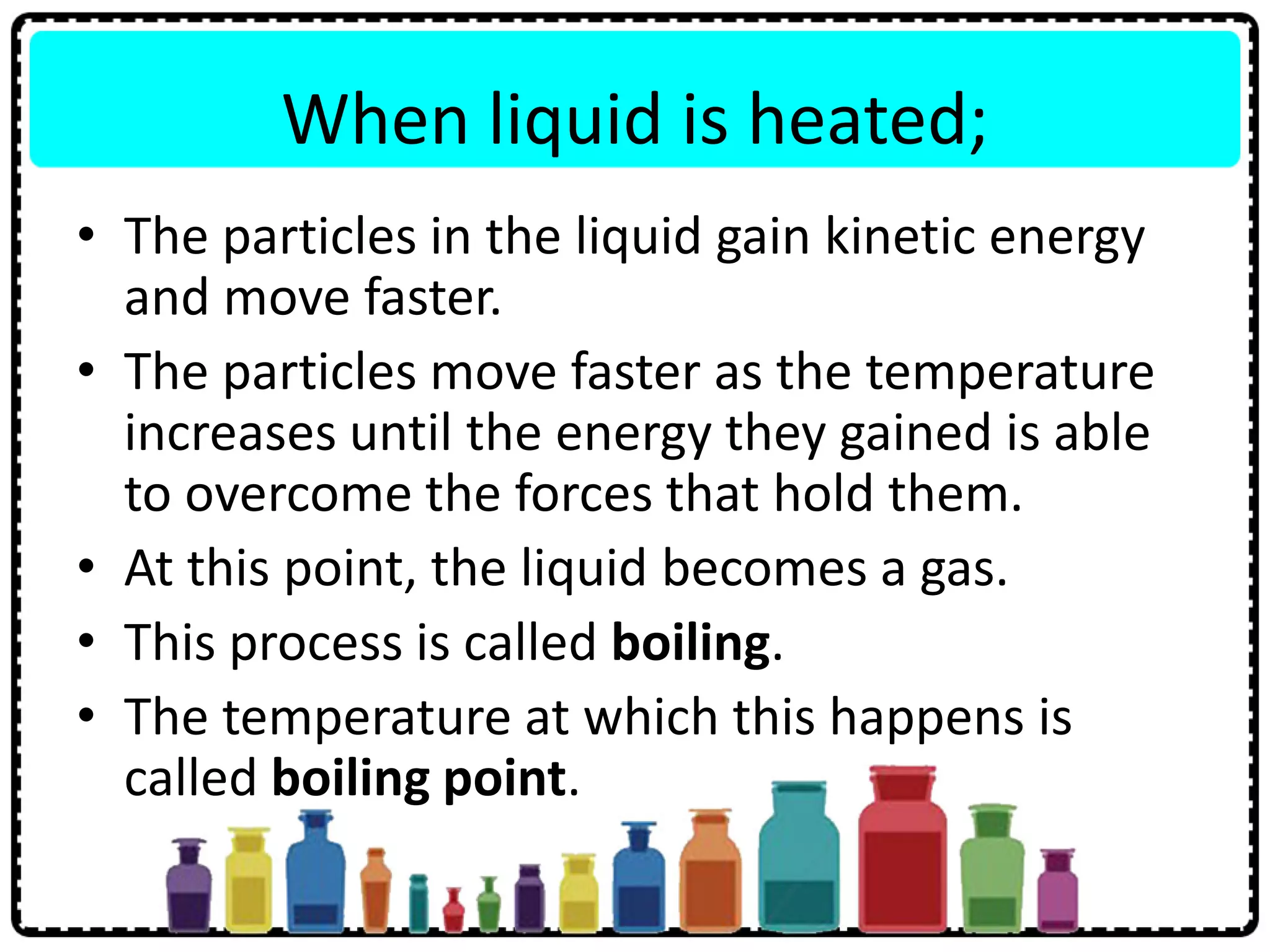
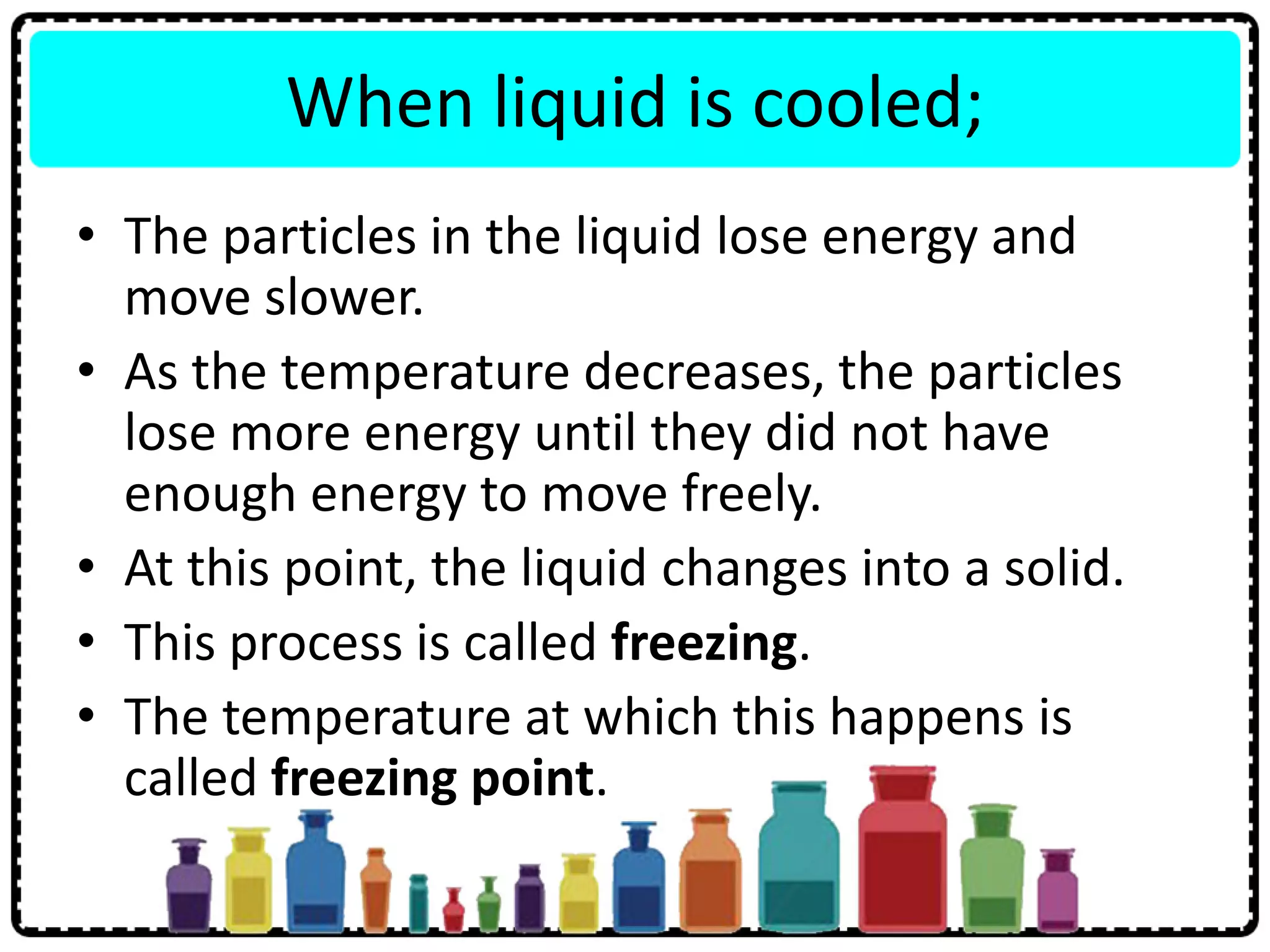
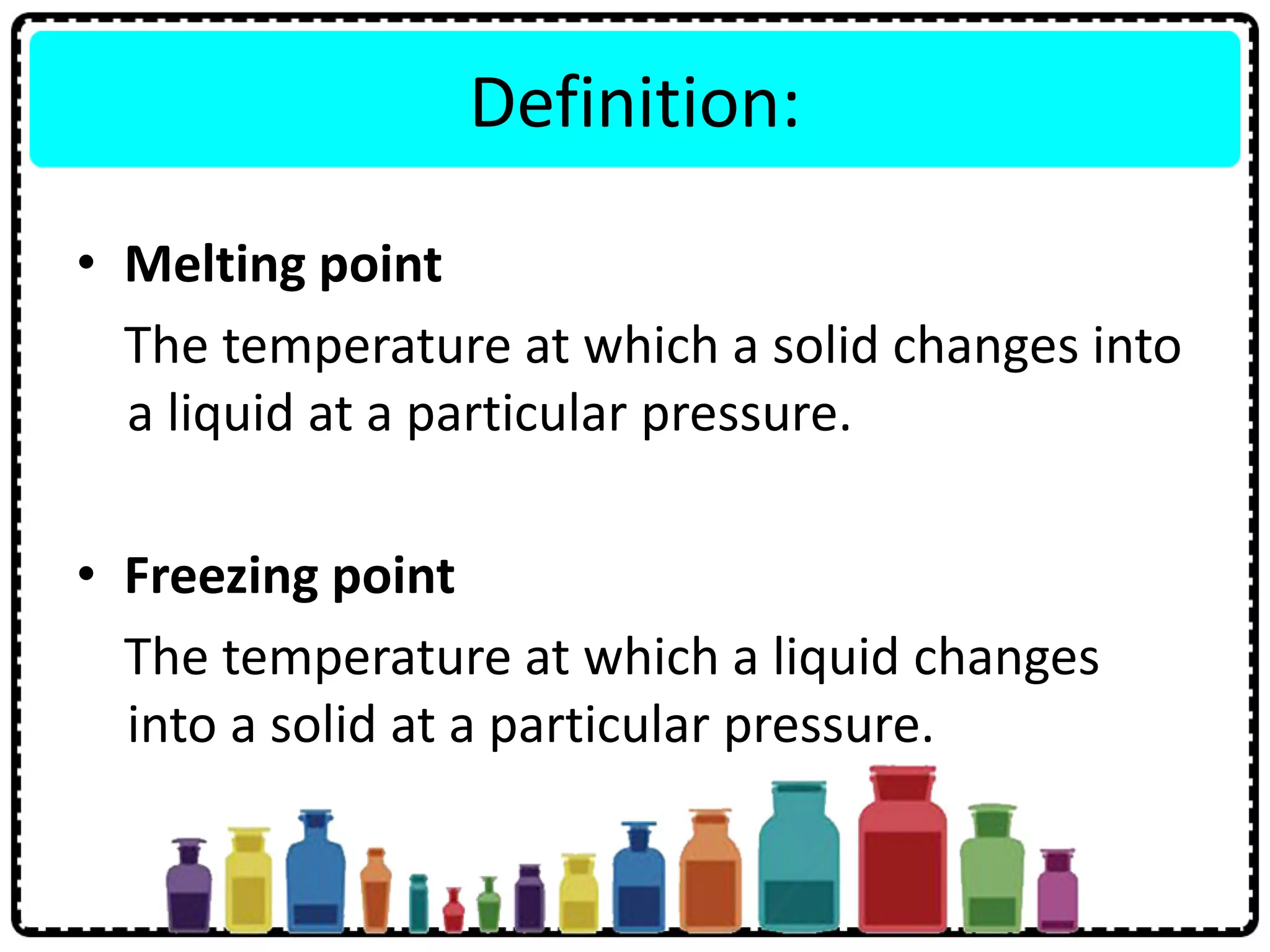
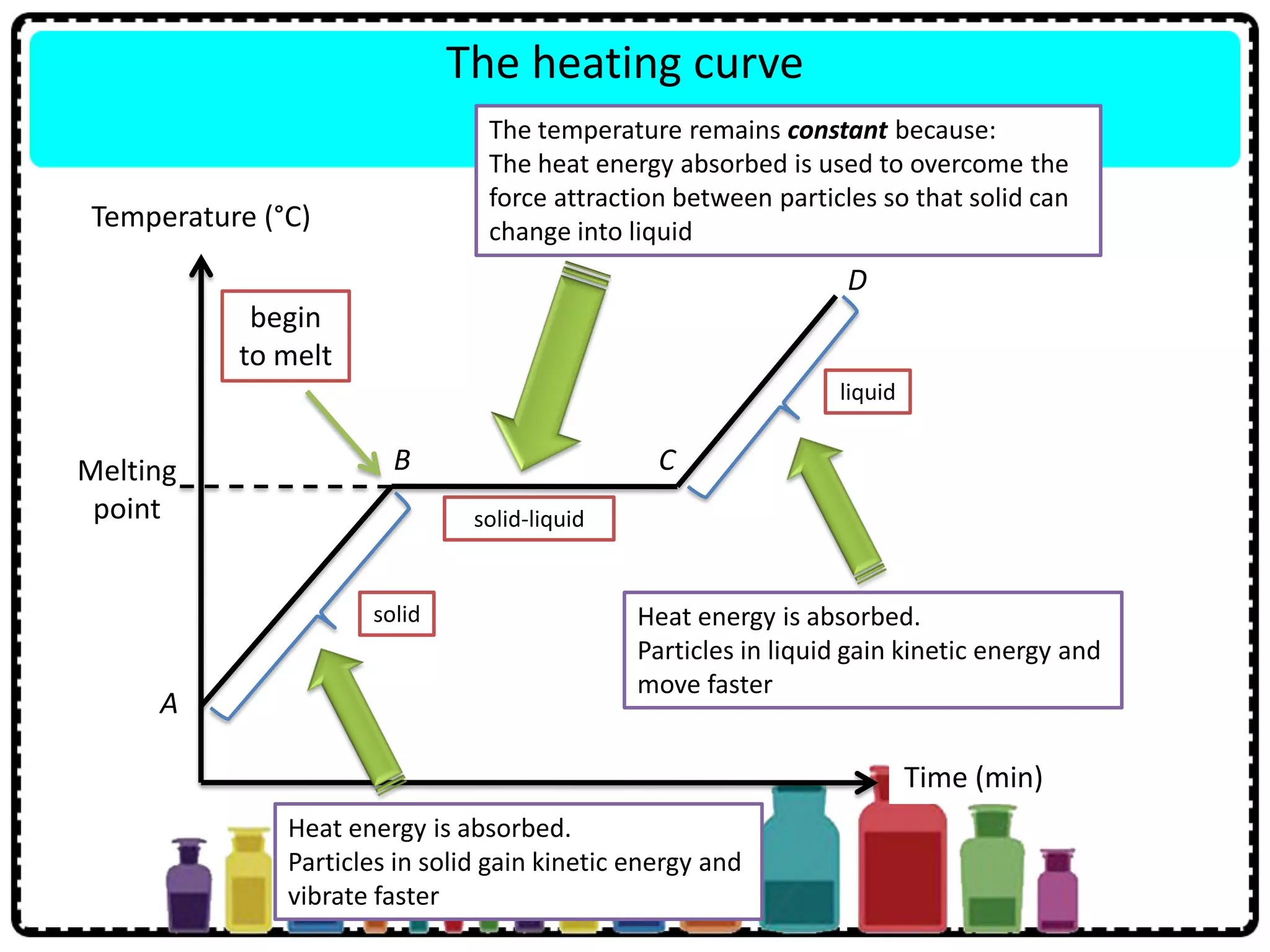
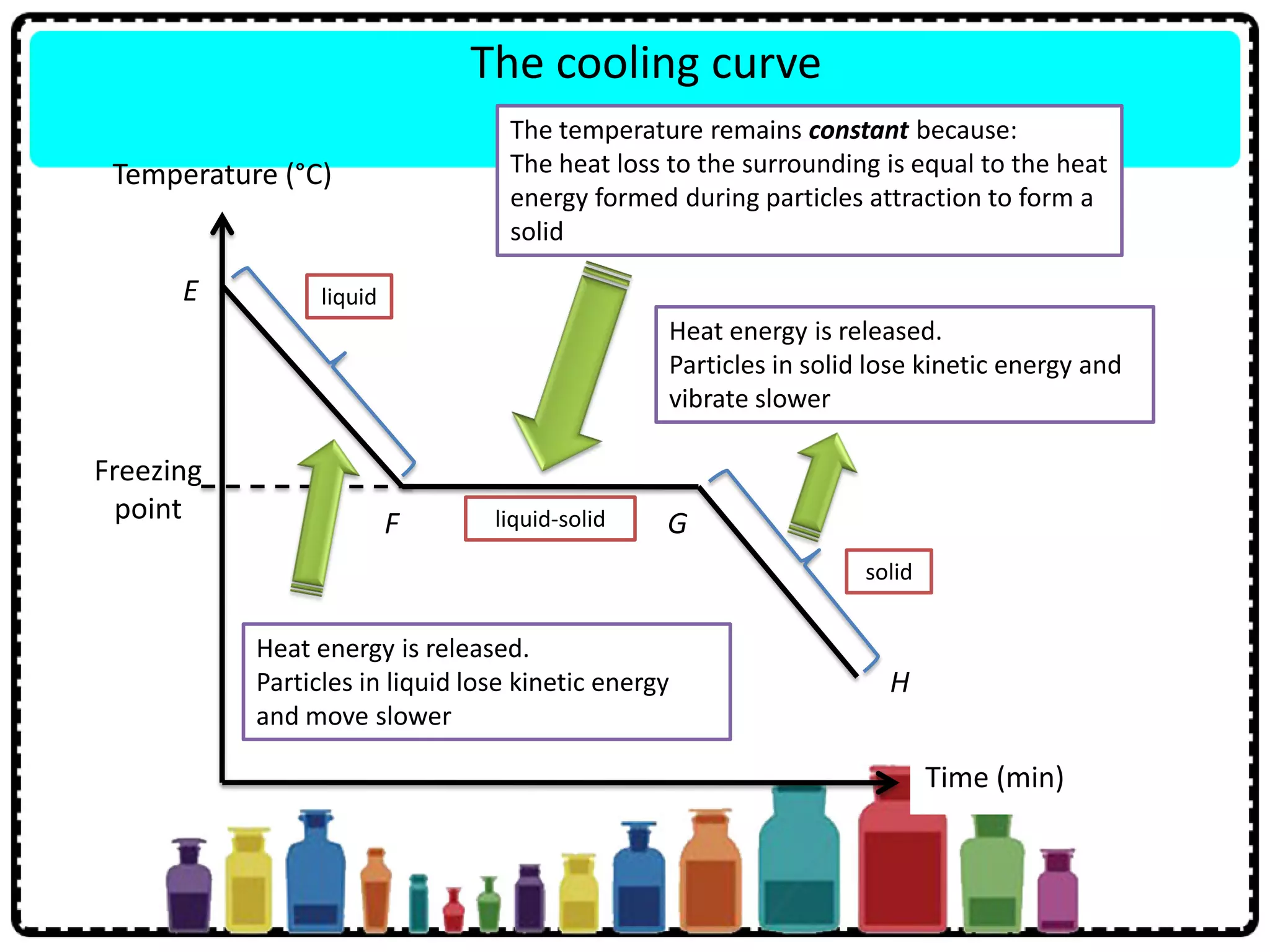
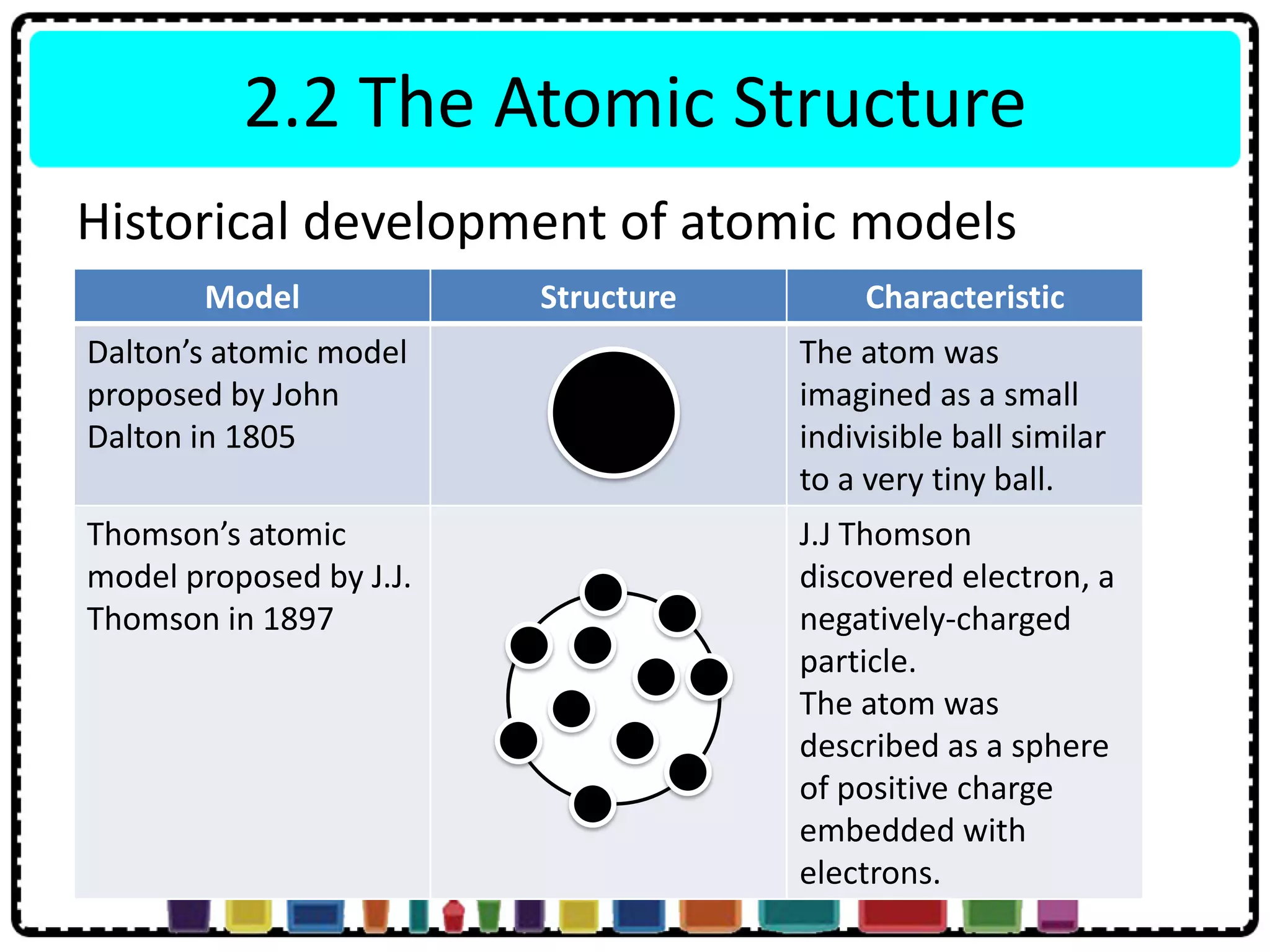
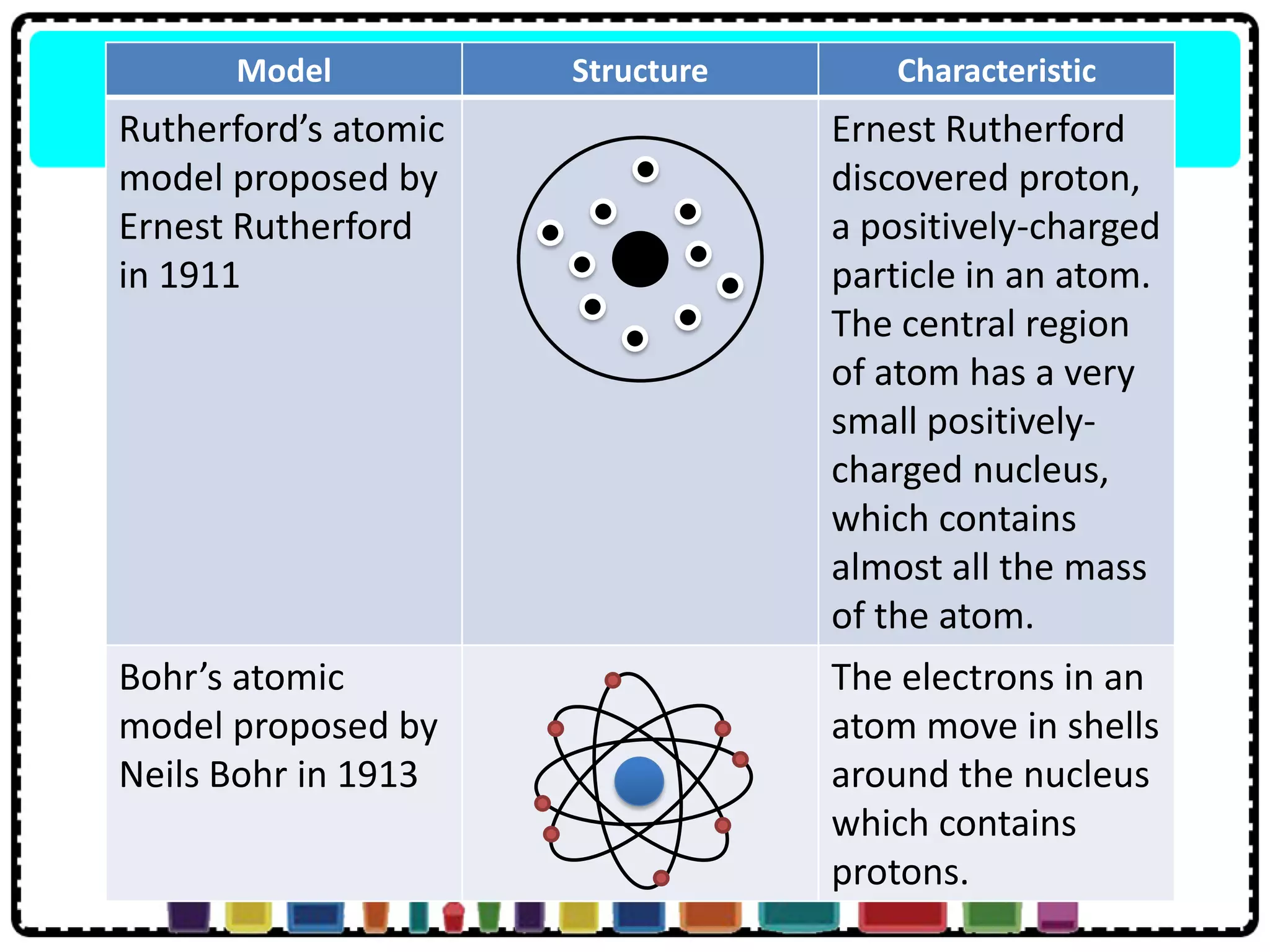
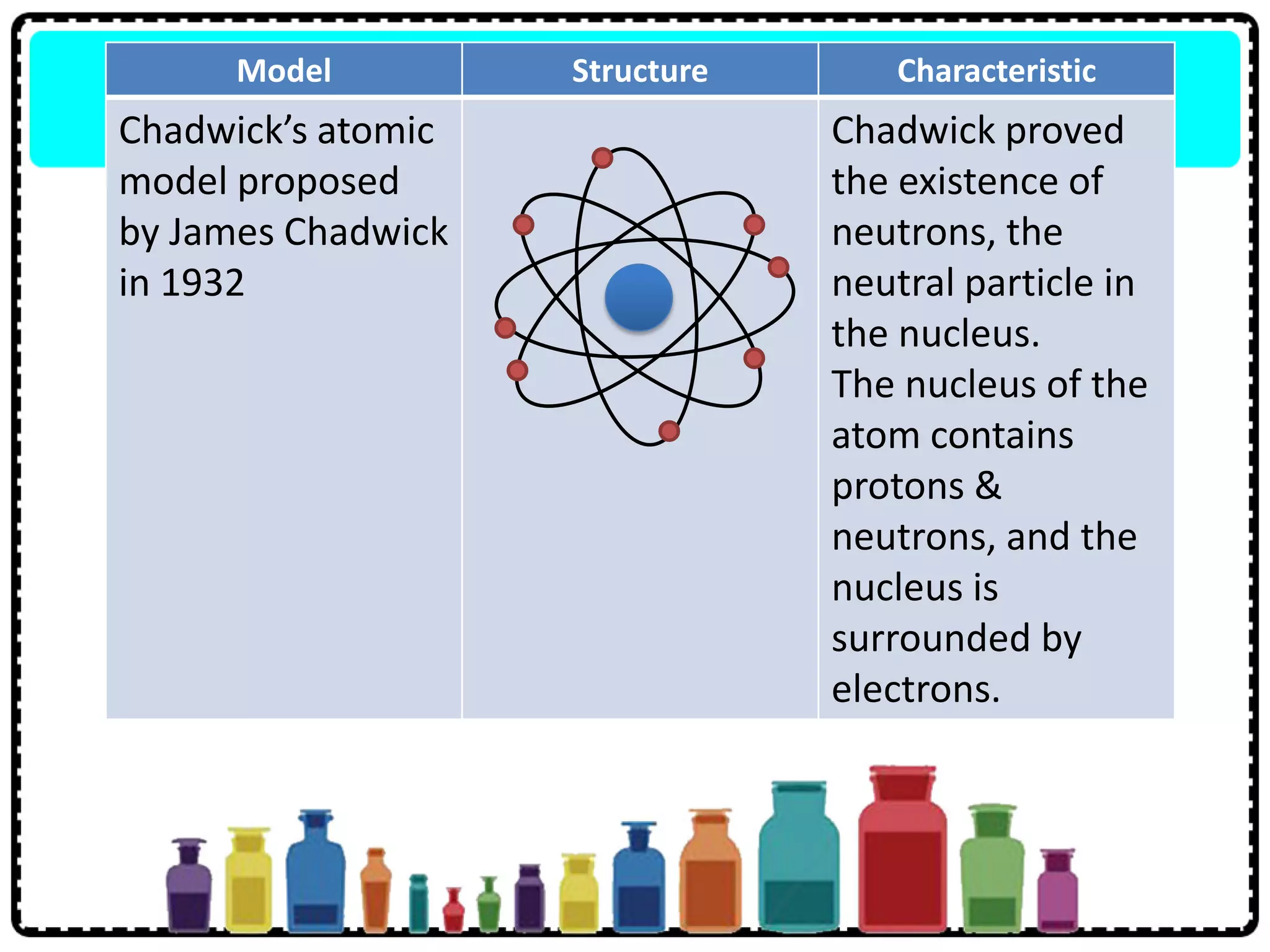
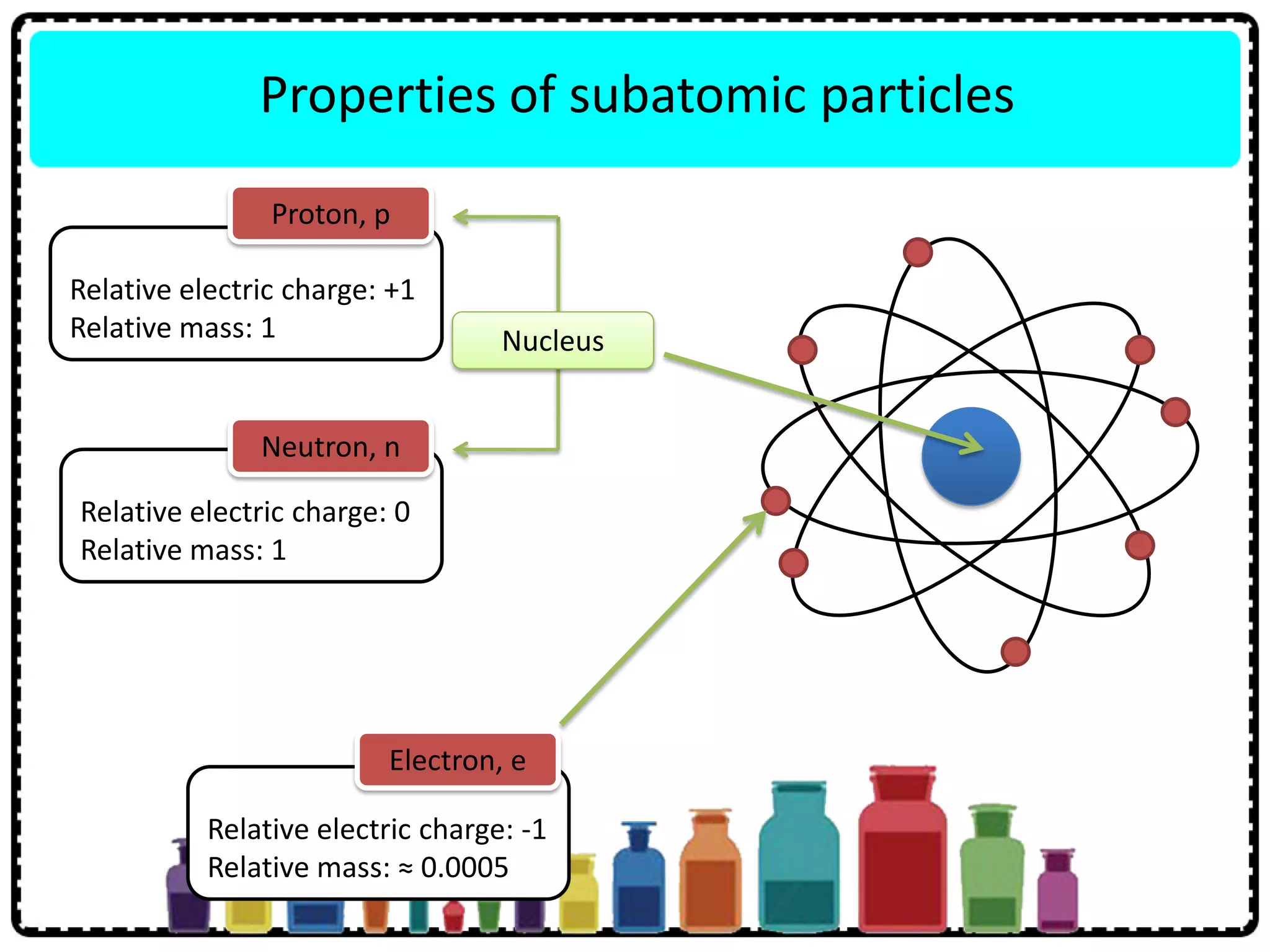
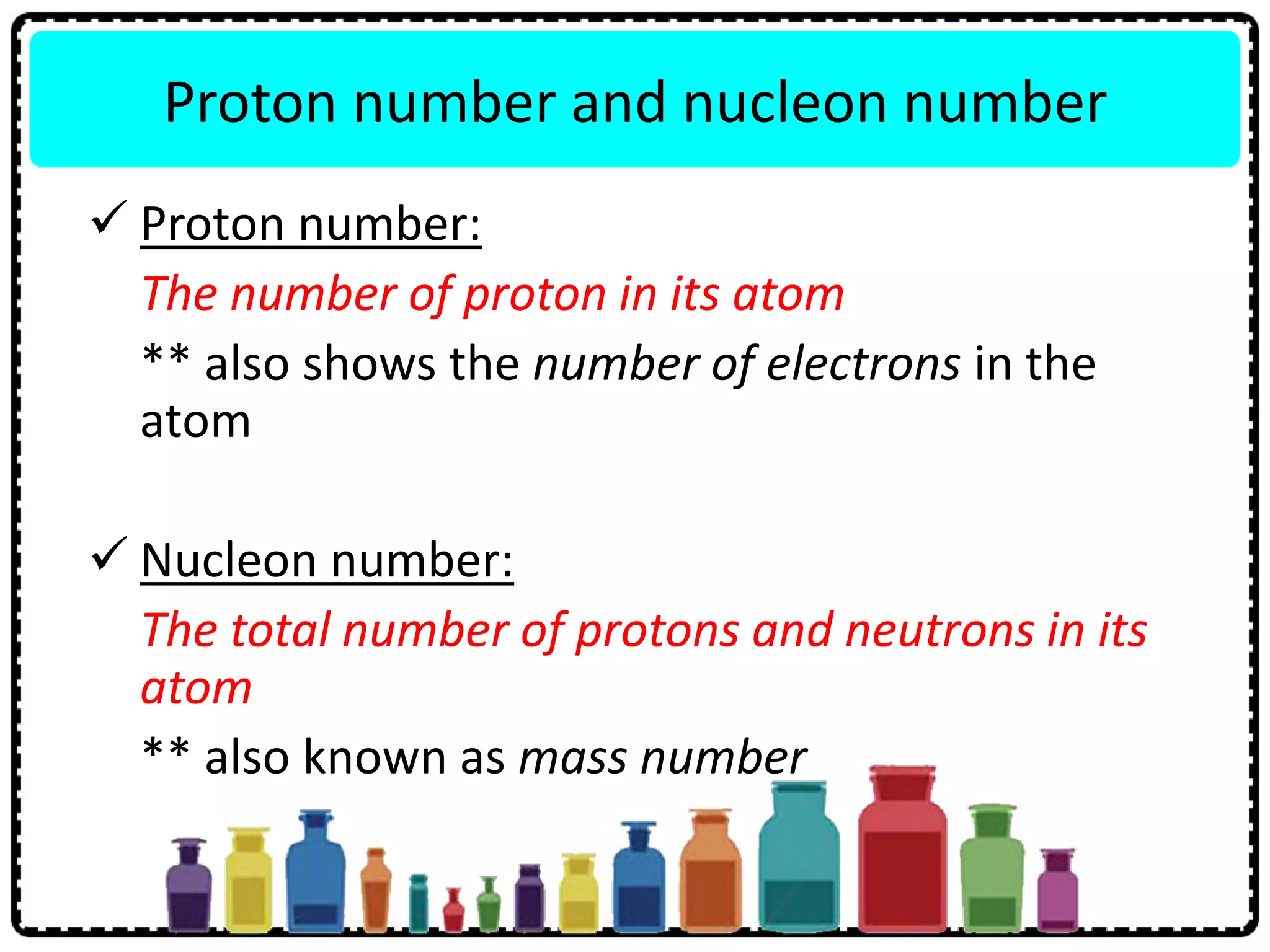
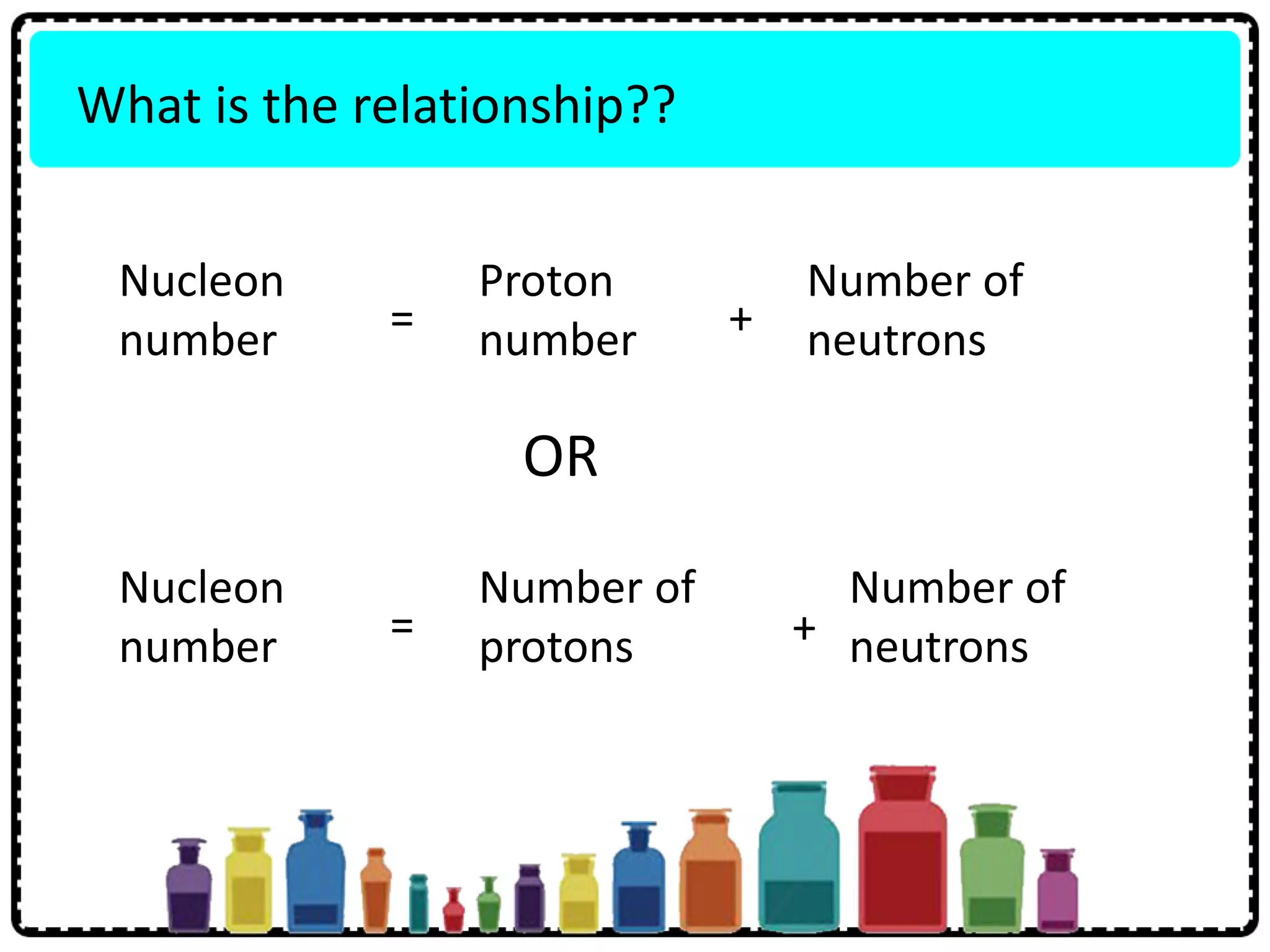
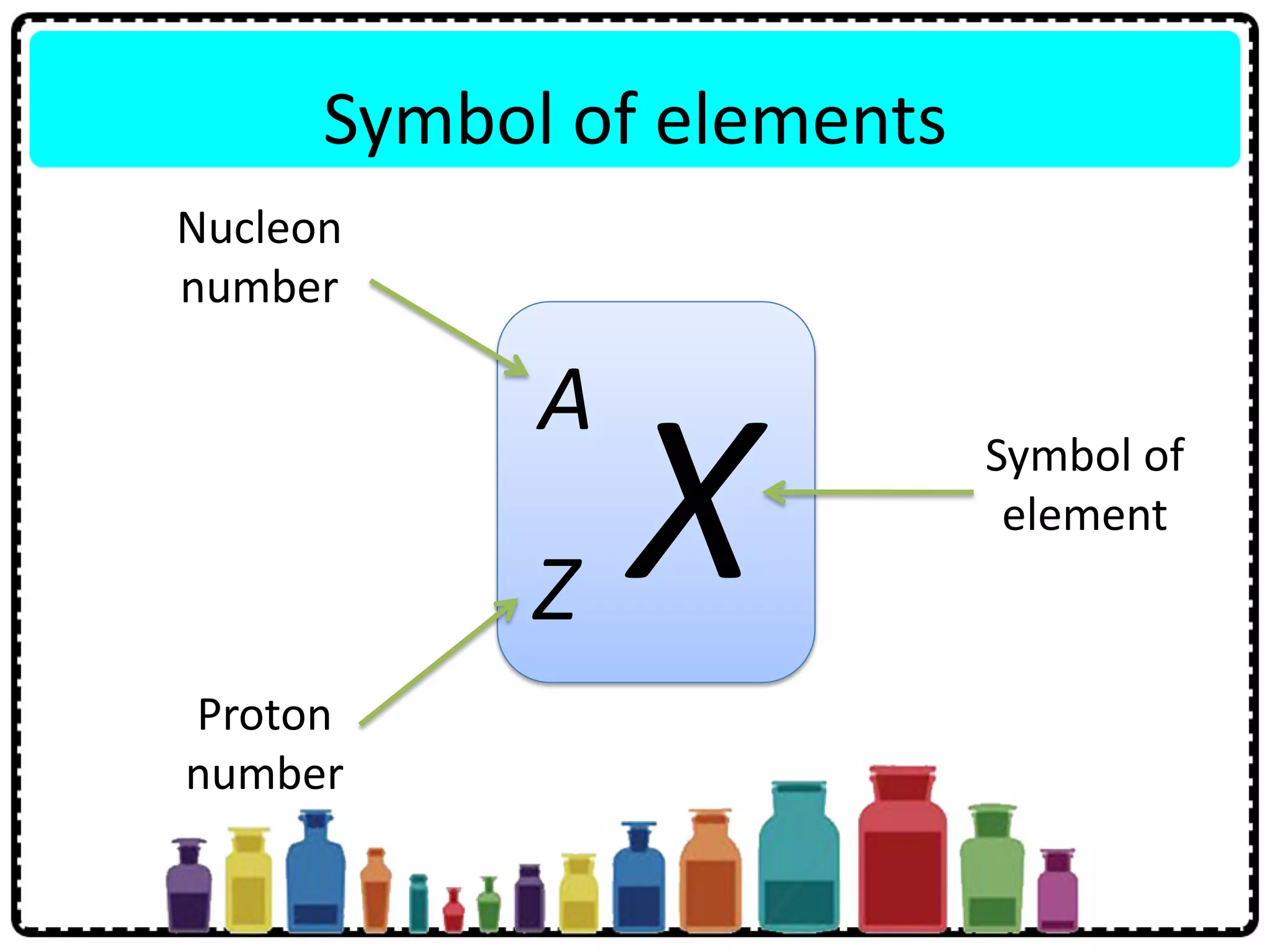
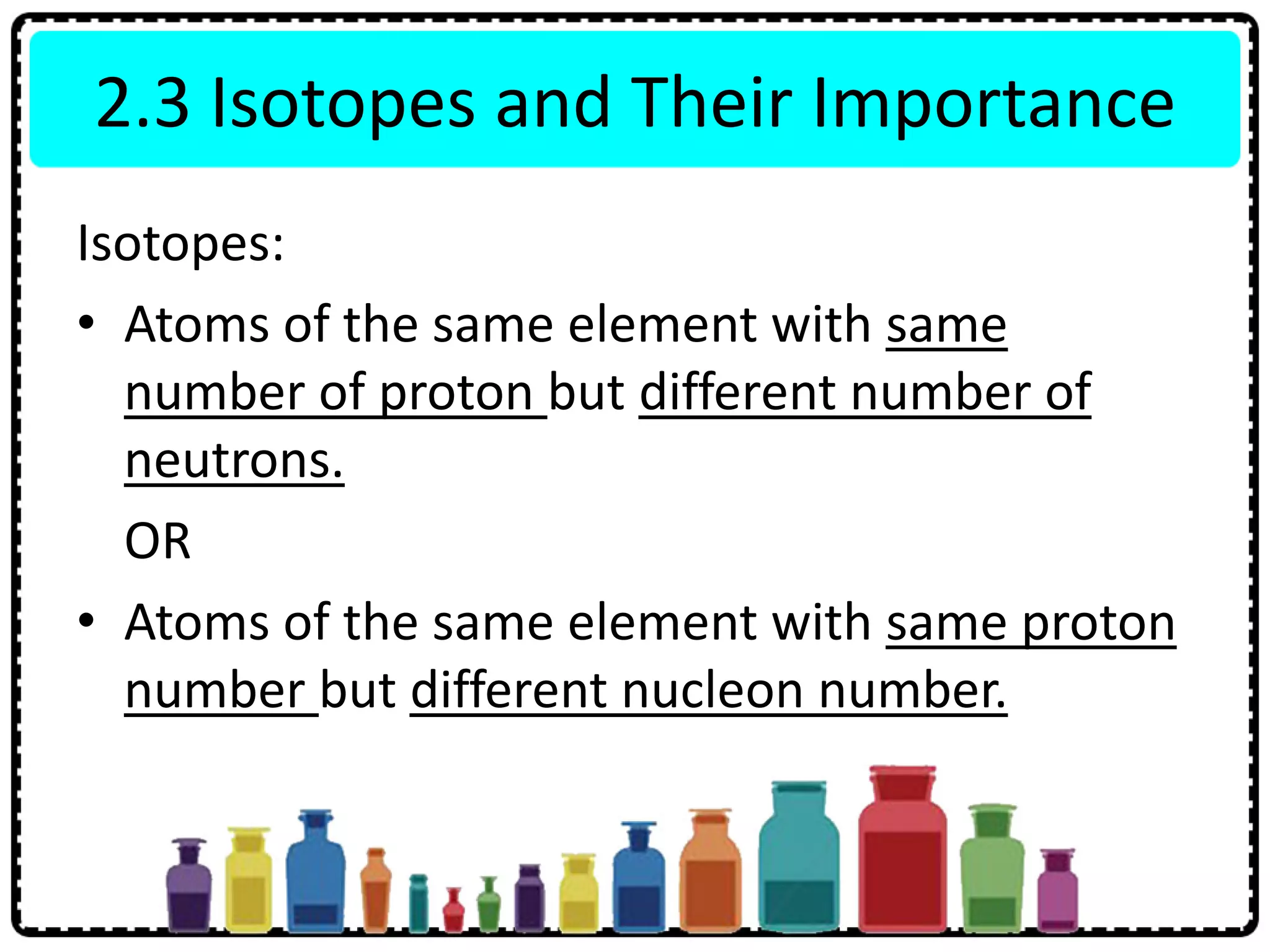
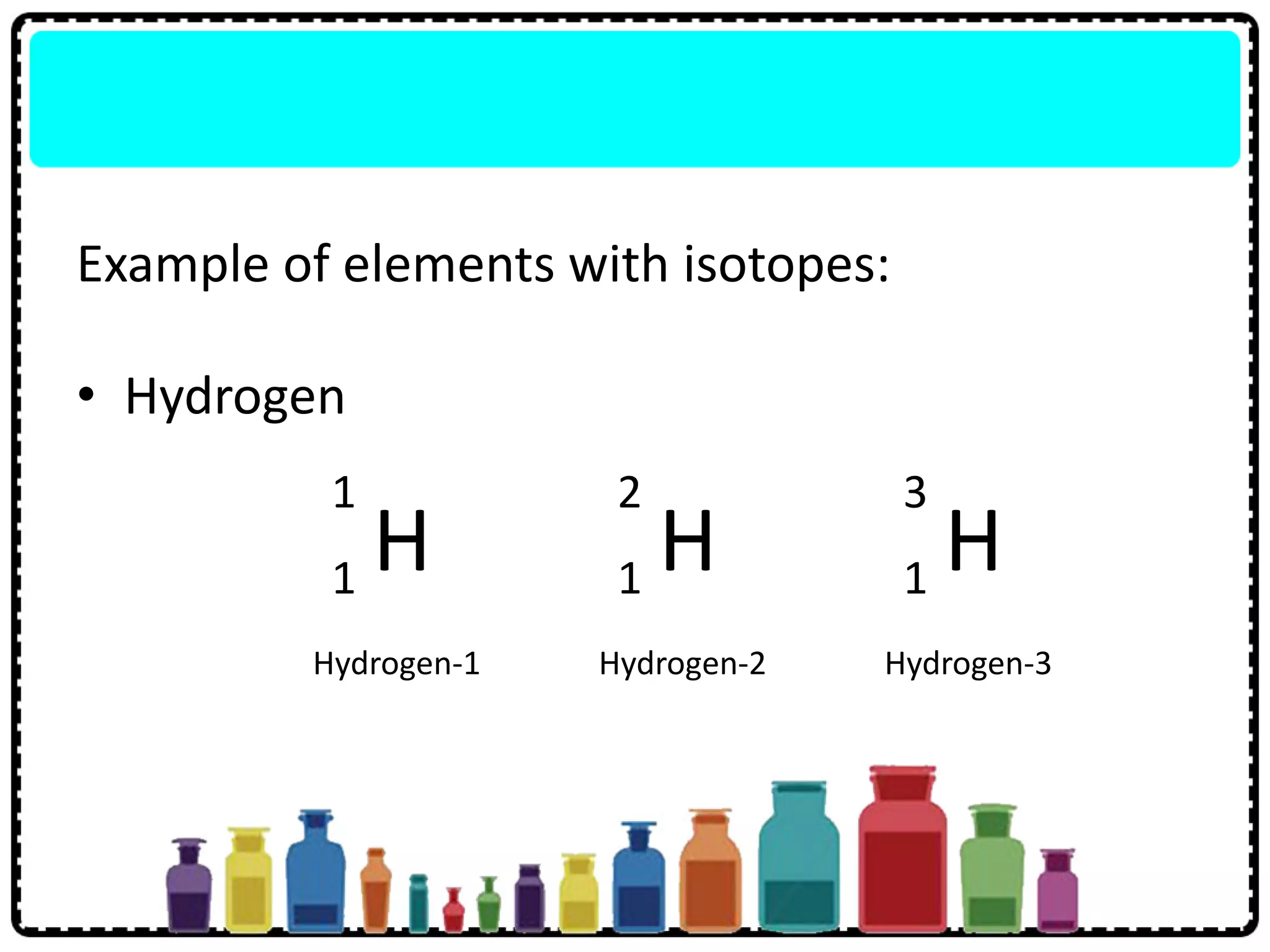
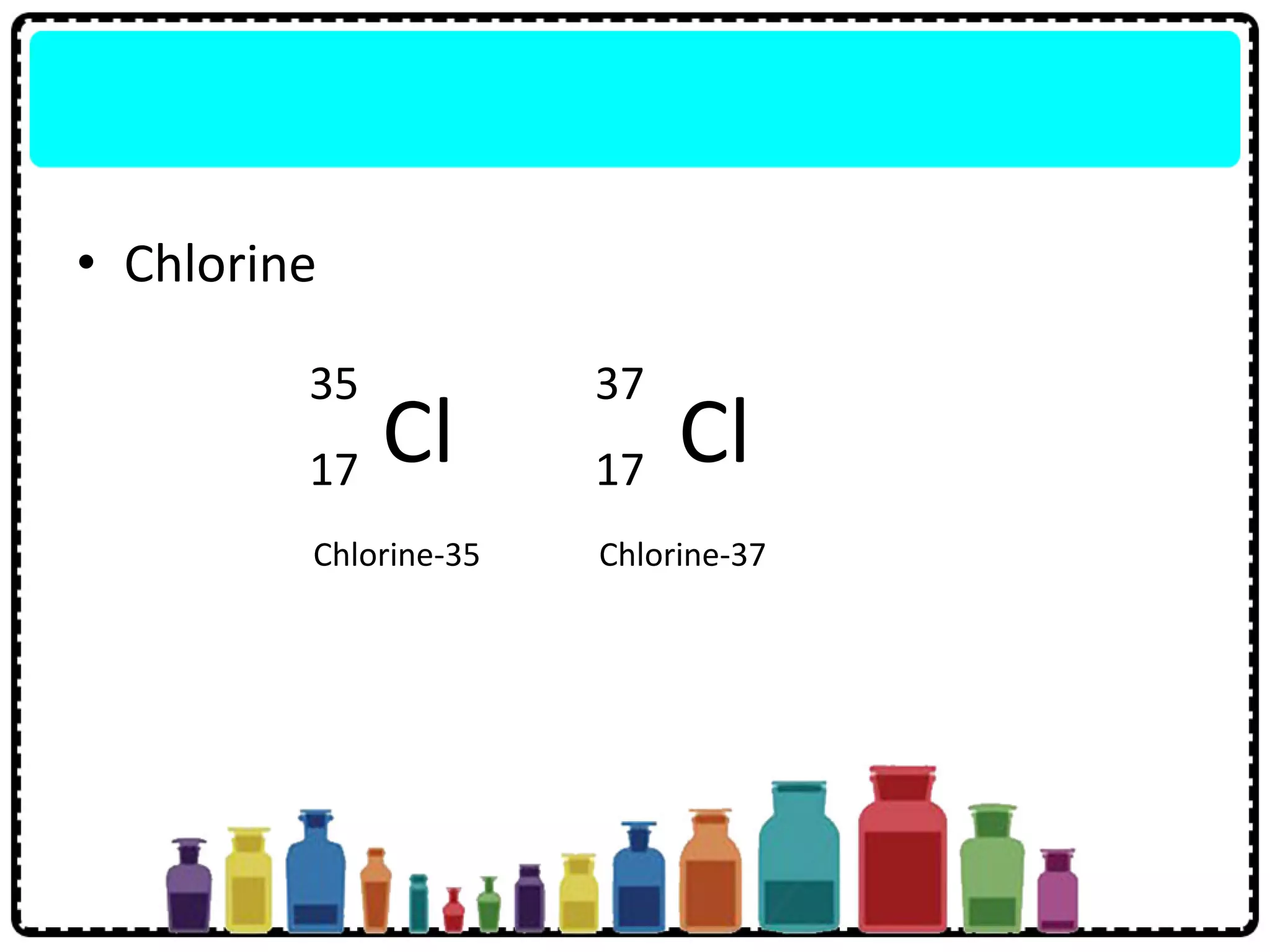
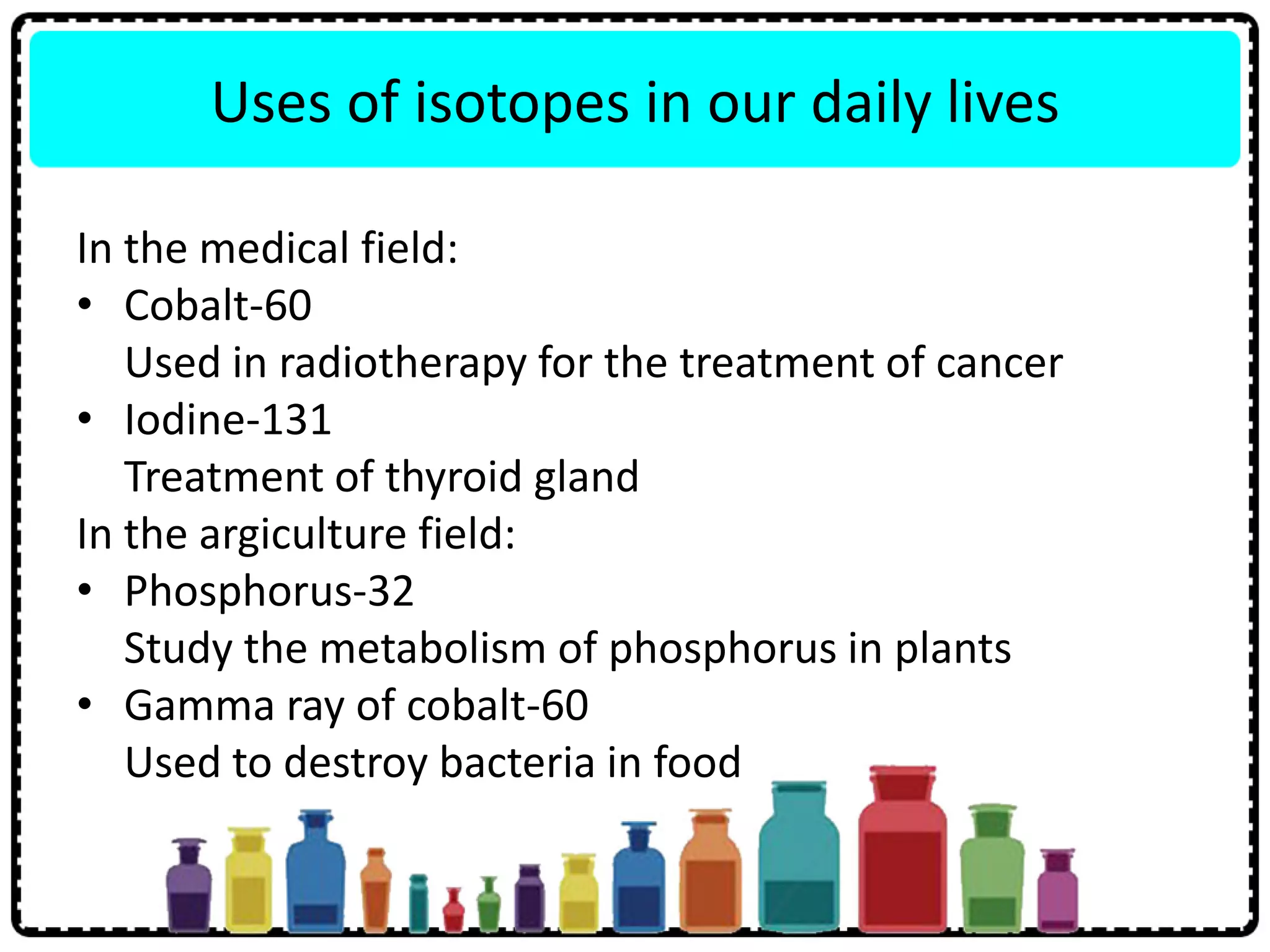
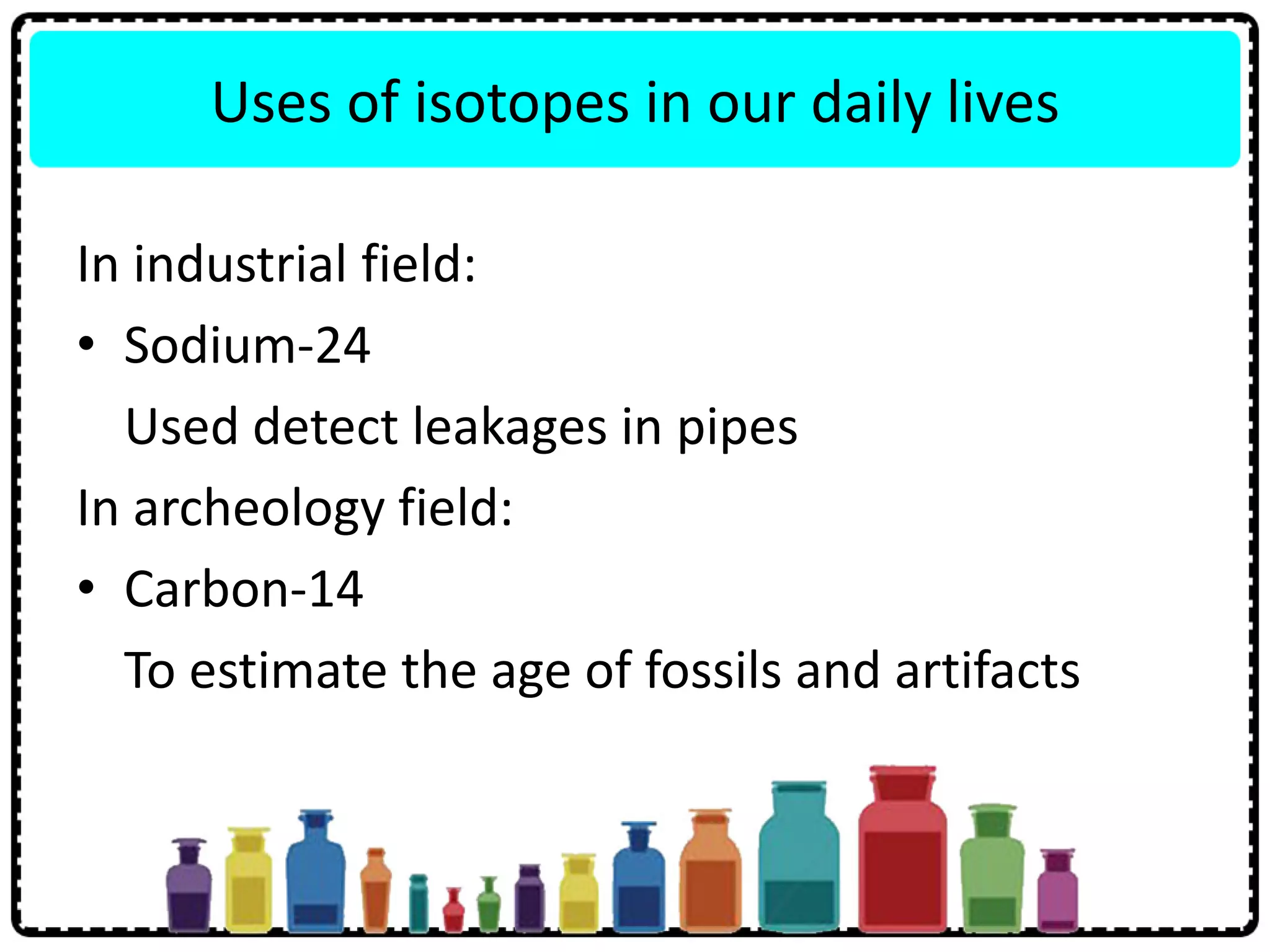
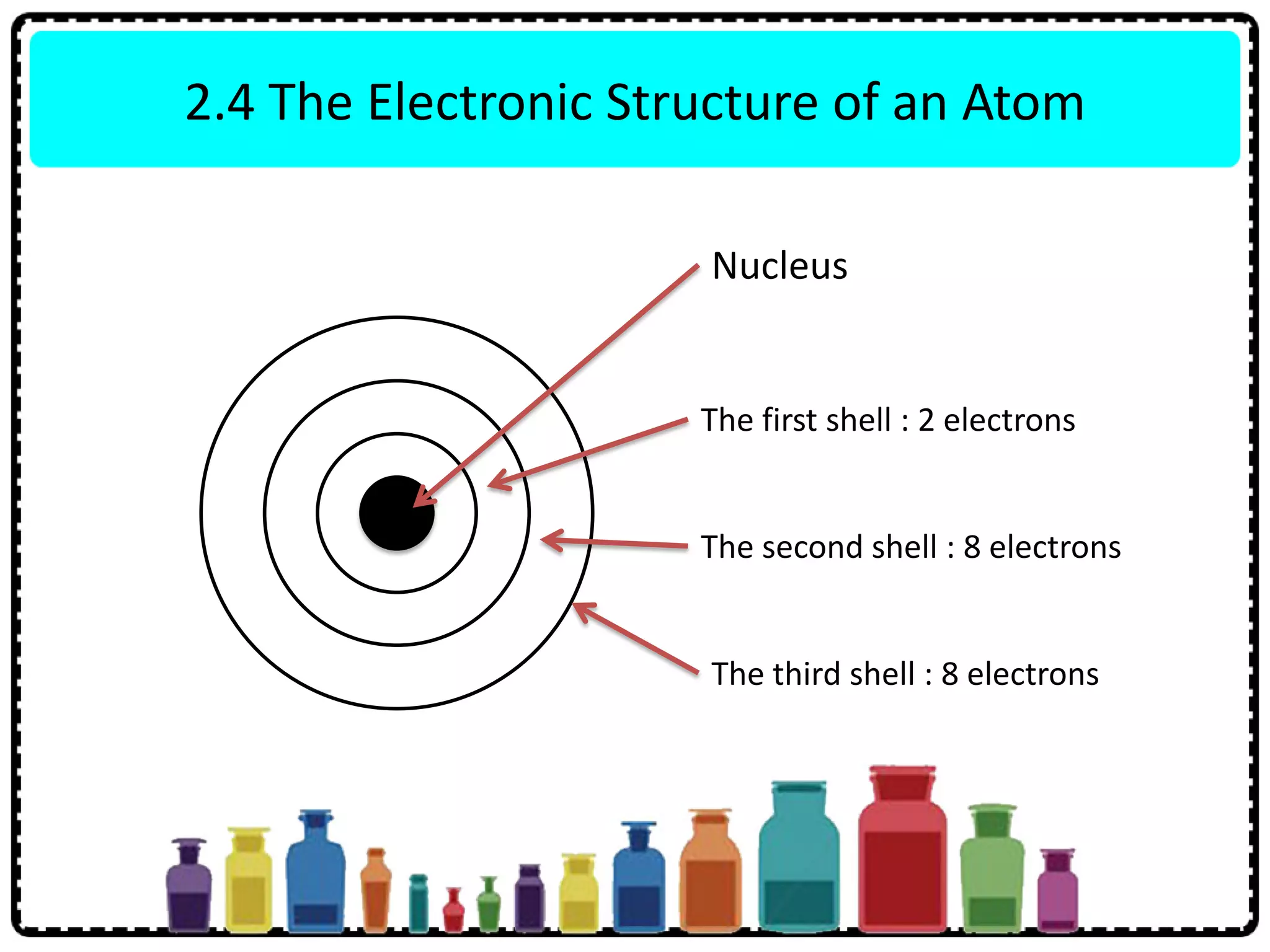
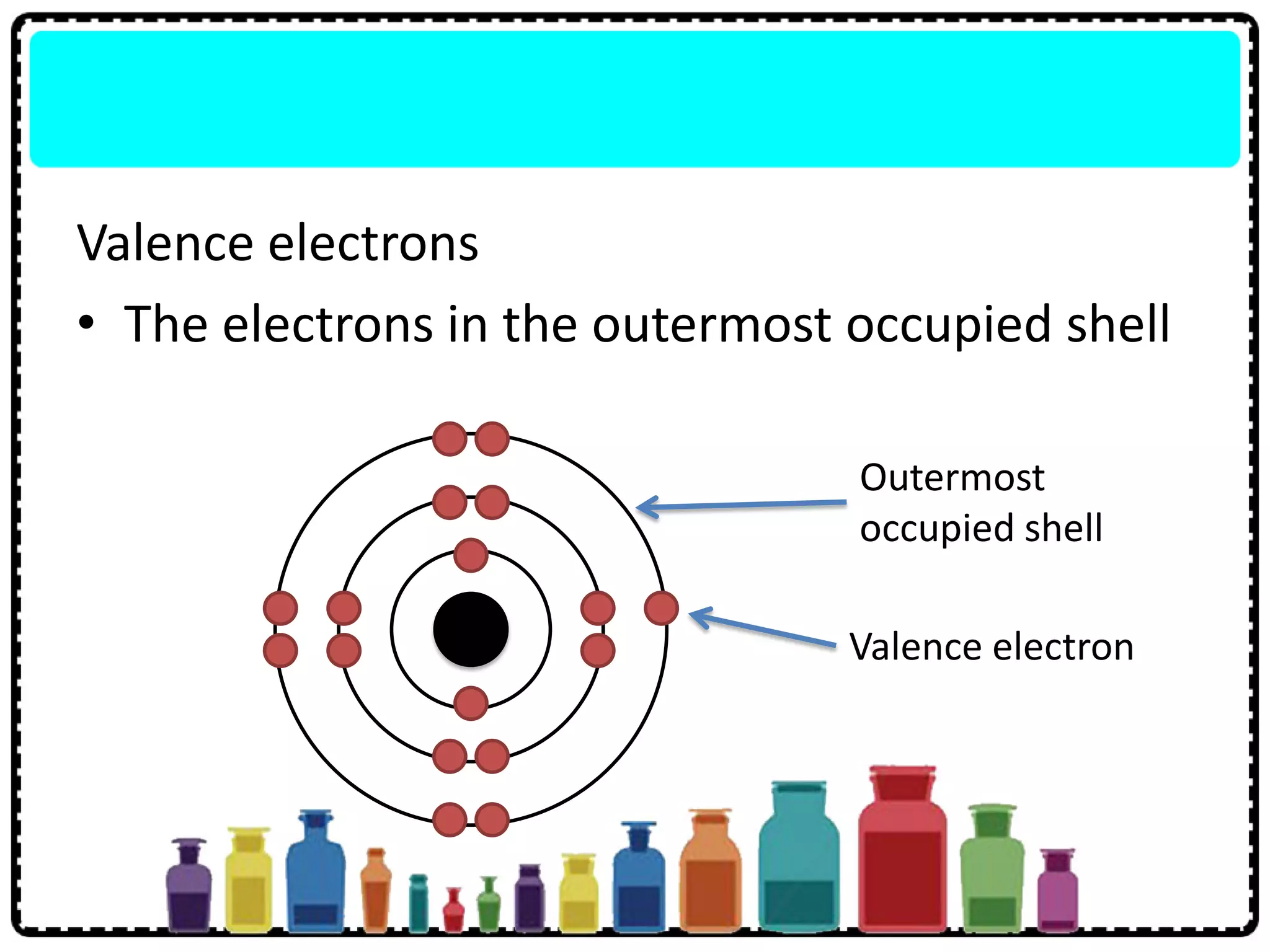
This document discusses the structure of atoms. It defines key terms like atoms, molecules, and ions. Atoms are made up of subatomic particles like protons, neutrons, and electrons. The document traces the historical development of atomic models from Dalton to Chadwick. Isotopes are defined as atoms of the same element with different numbers of neutrons. Examples of isotopes in hydrogen and chlorine are given. Uses of isotopes in medicine, agriculture, and other fields are outlined. The electronic structure of atoms is explained using the example of chlorine's 2.8.7 configuration and defining valence electrons.