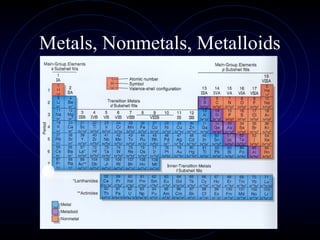
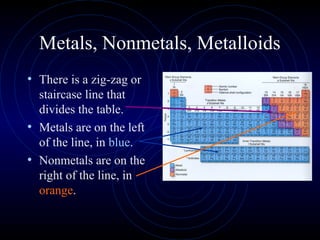
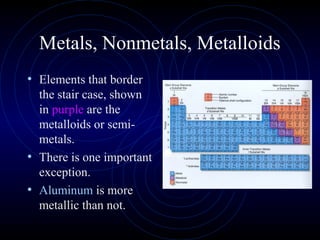
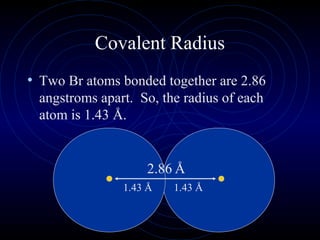
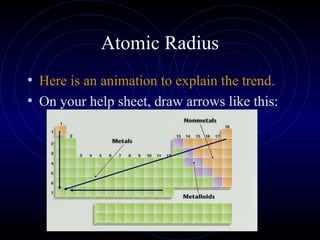
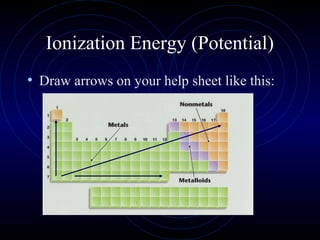
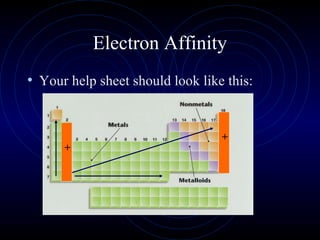
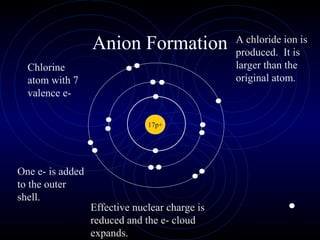
The document discusses periodic trends in elemental properties. It explains that Dmitri Mendeleev was the first to organize elements in a periodic table based on their properties. Elements in the same group have similar properties due to their valence electrons. Atomic radius generally decreases moving left to right across a period and increases moving down a group due to electron shielding. Ionization energy increases as atomic radius decreases. Electron affinity is exothermic when gaining electrons fills an orbital. Metallic character decreases and electronegativity increases moving from left to right. Cations are smaller than their parent atoms while anions are larger.