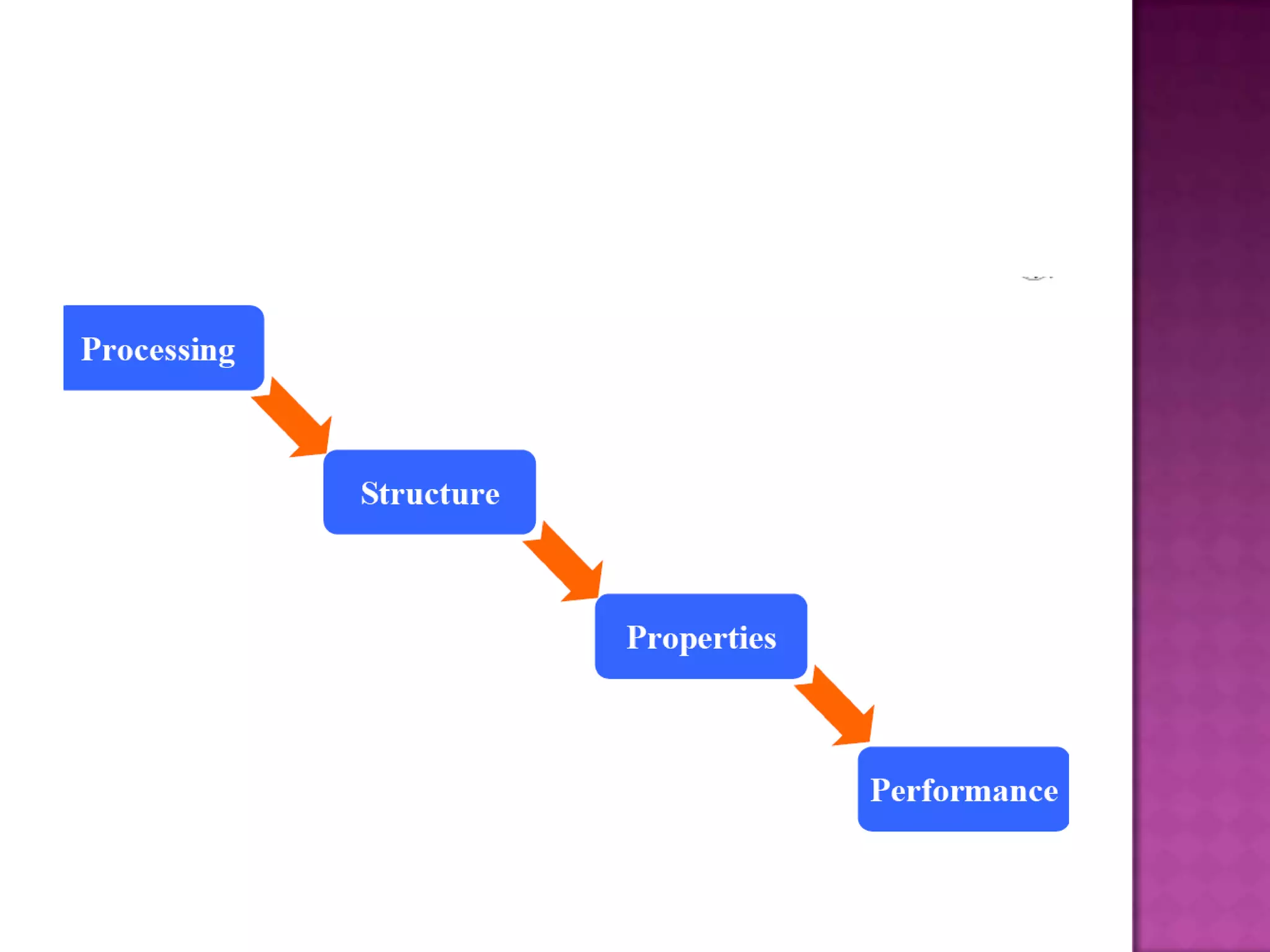
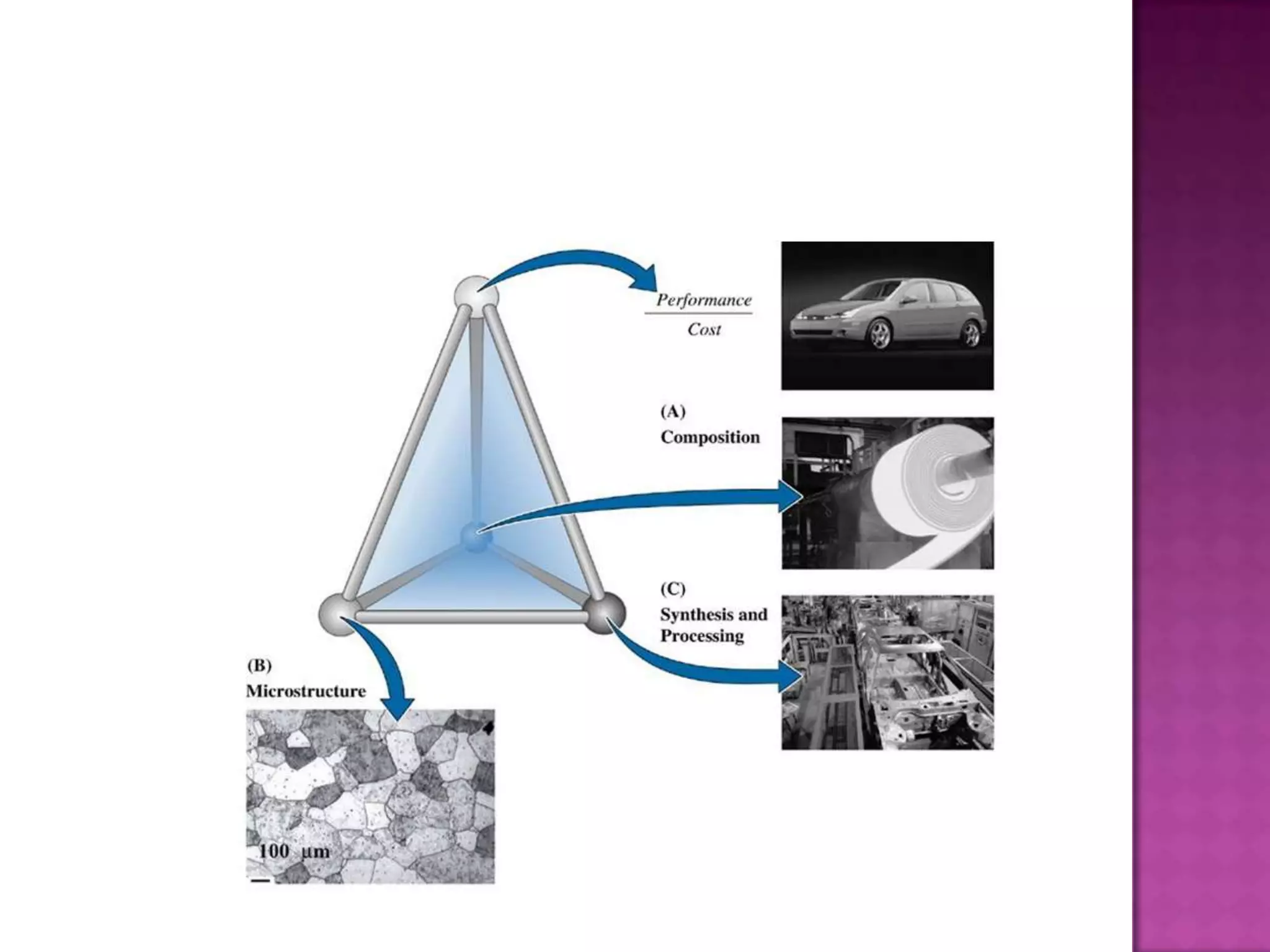
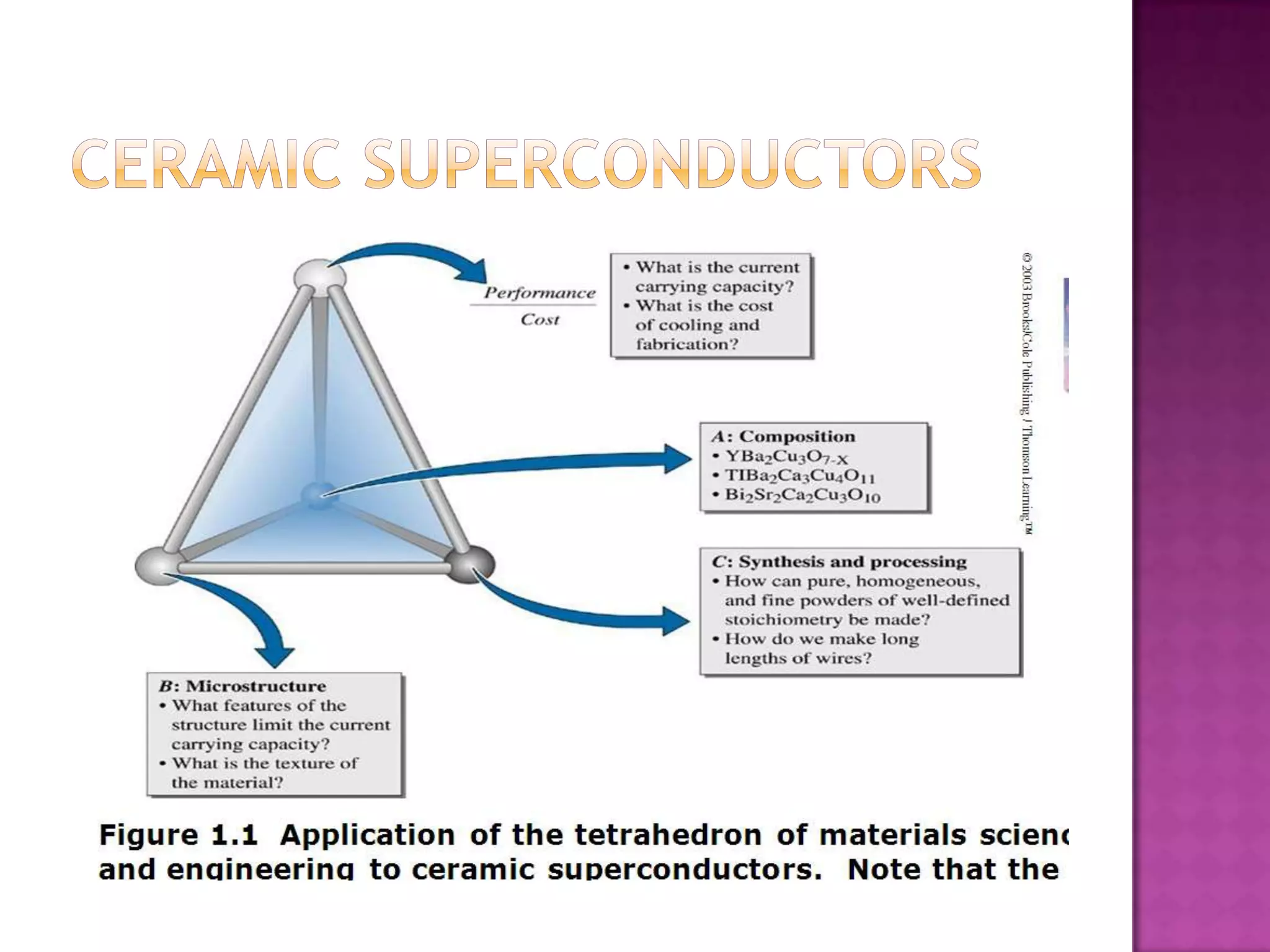
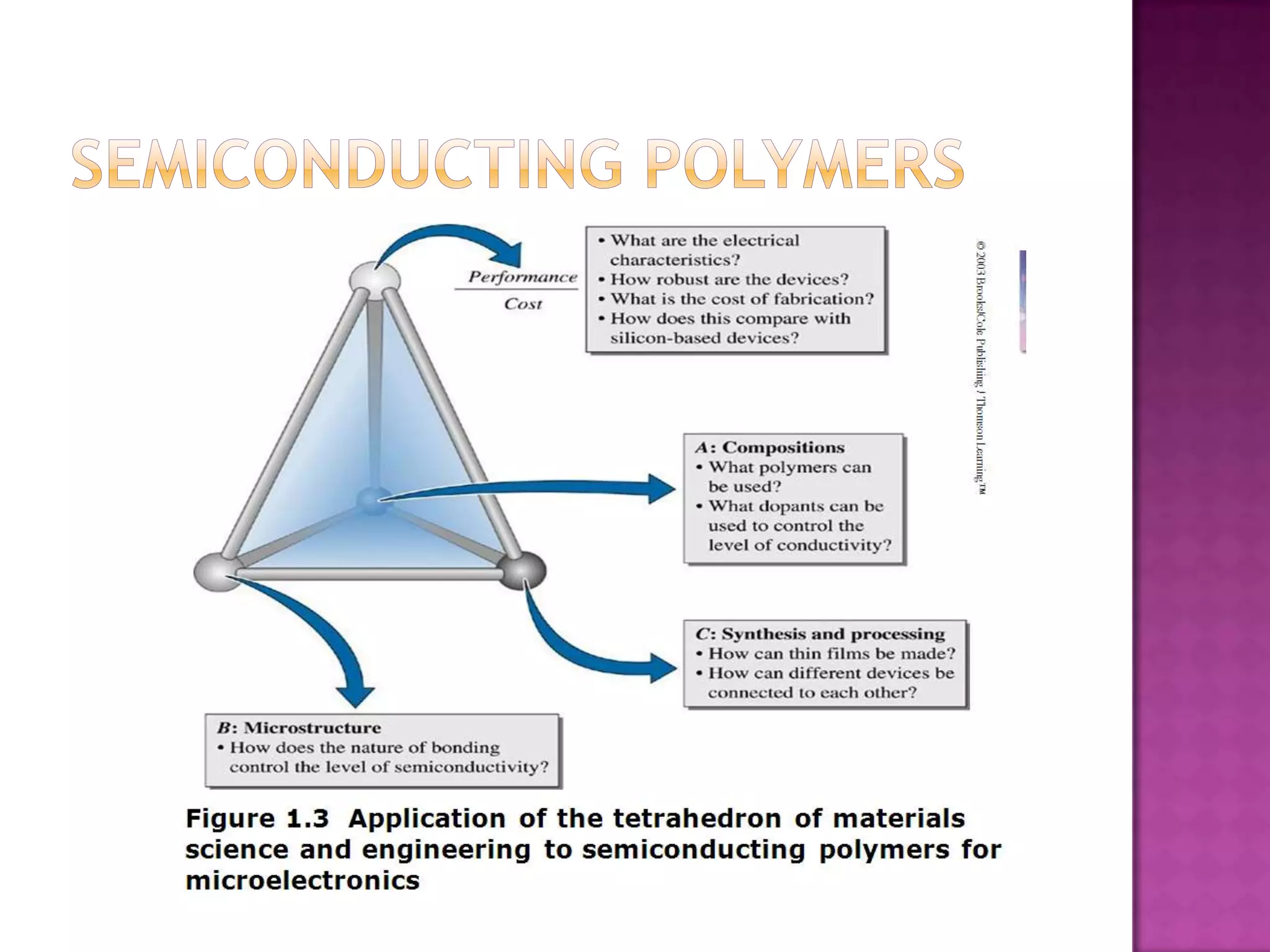
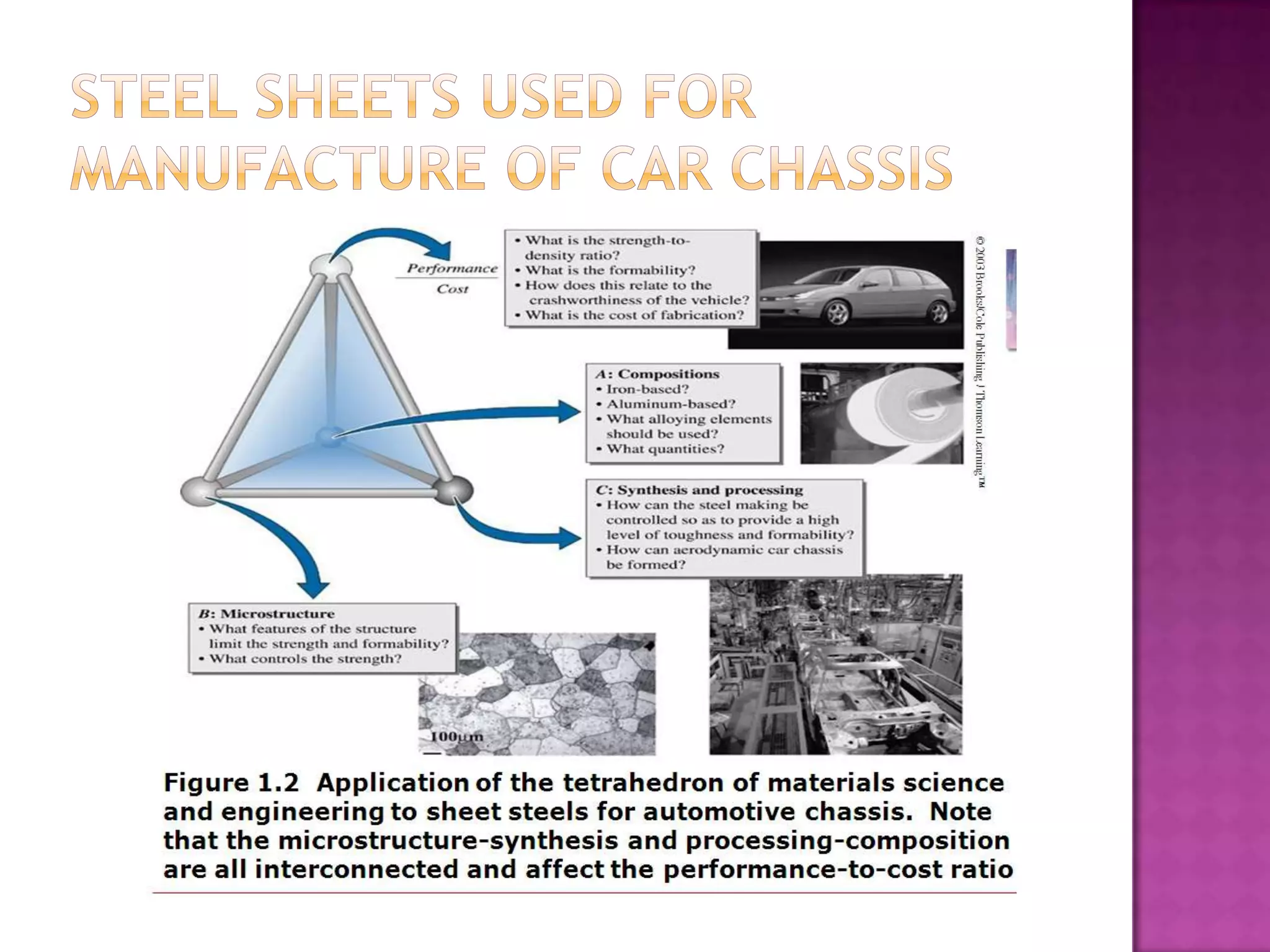
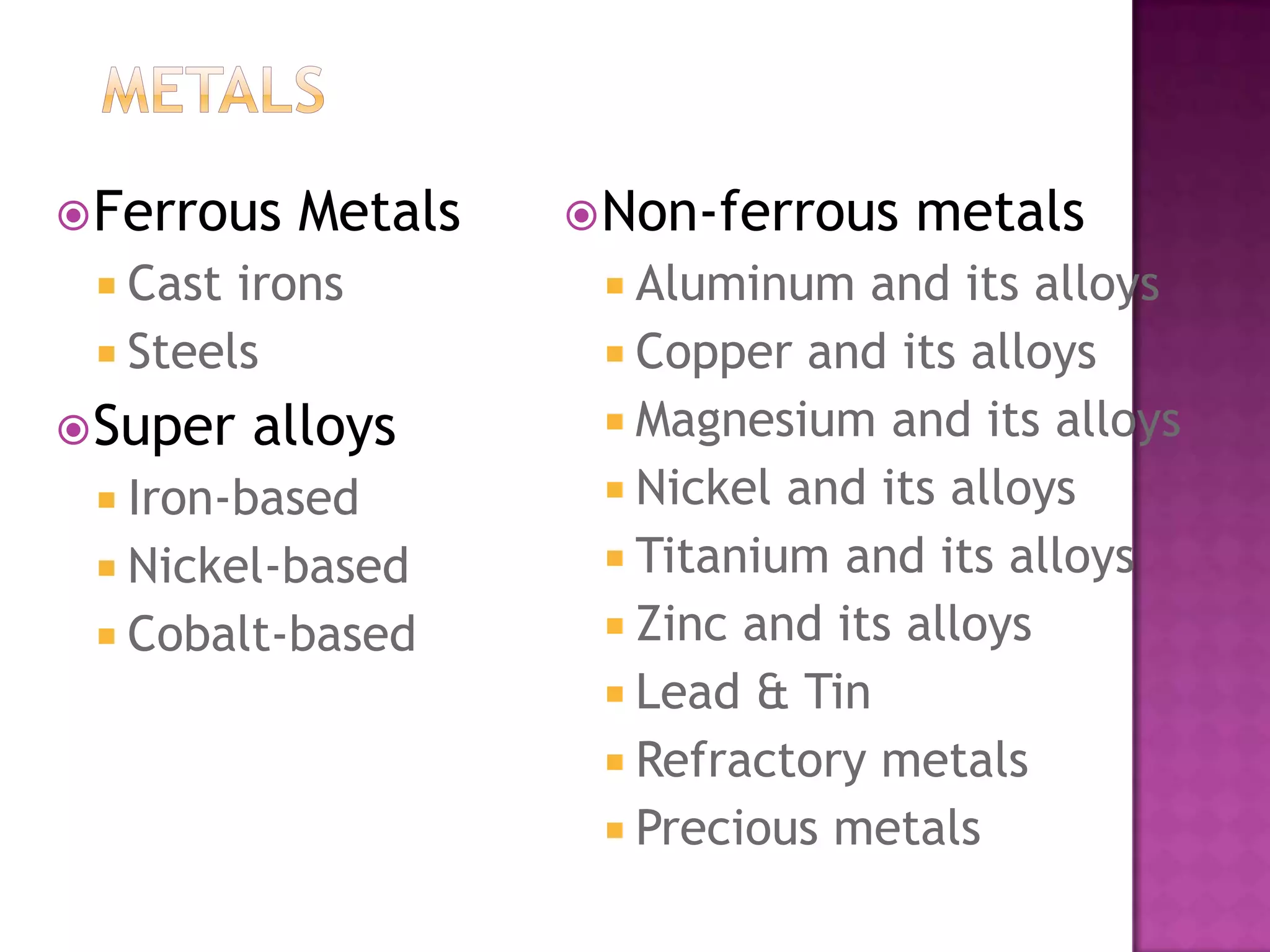
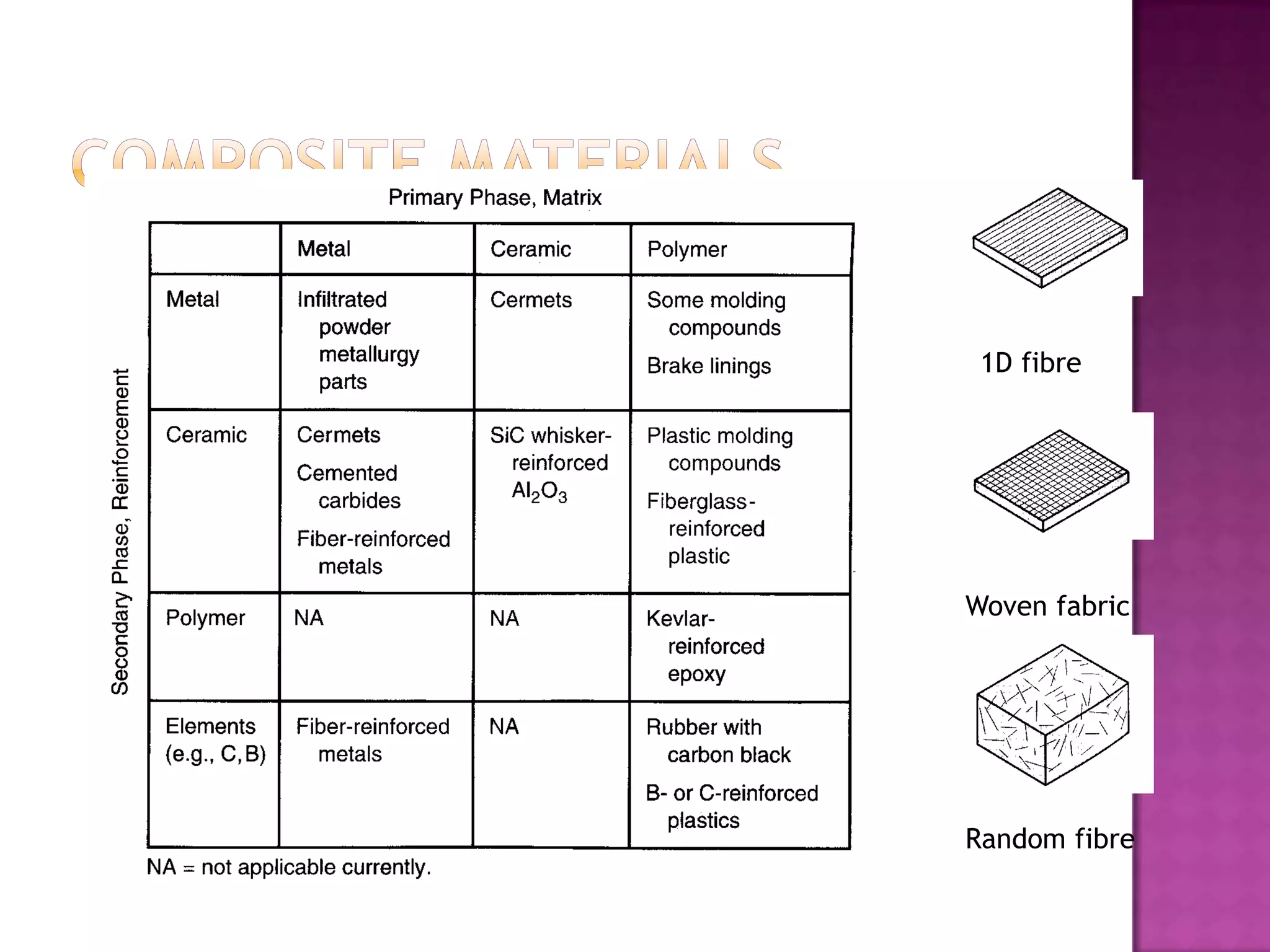
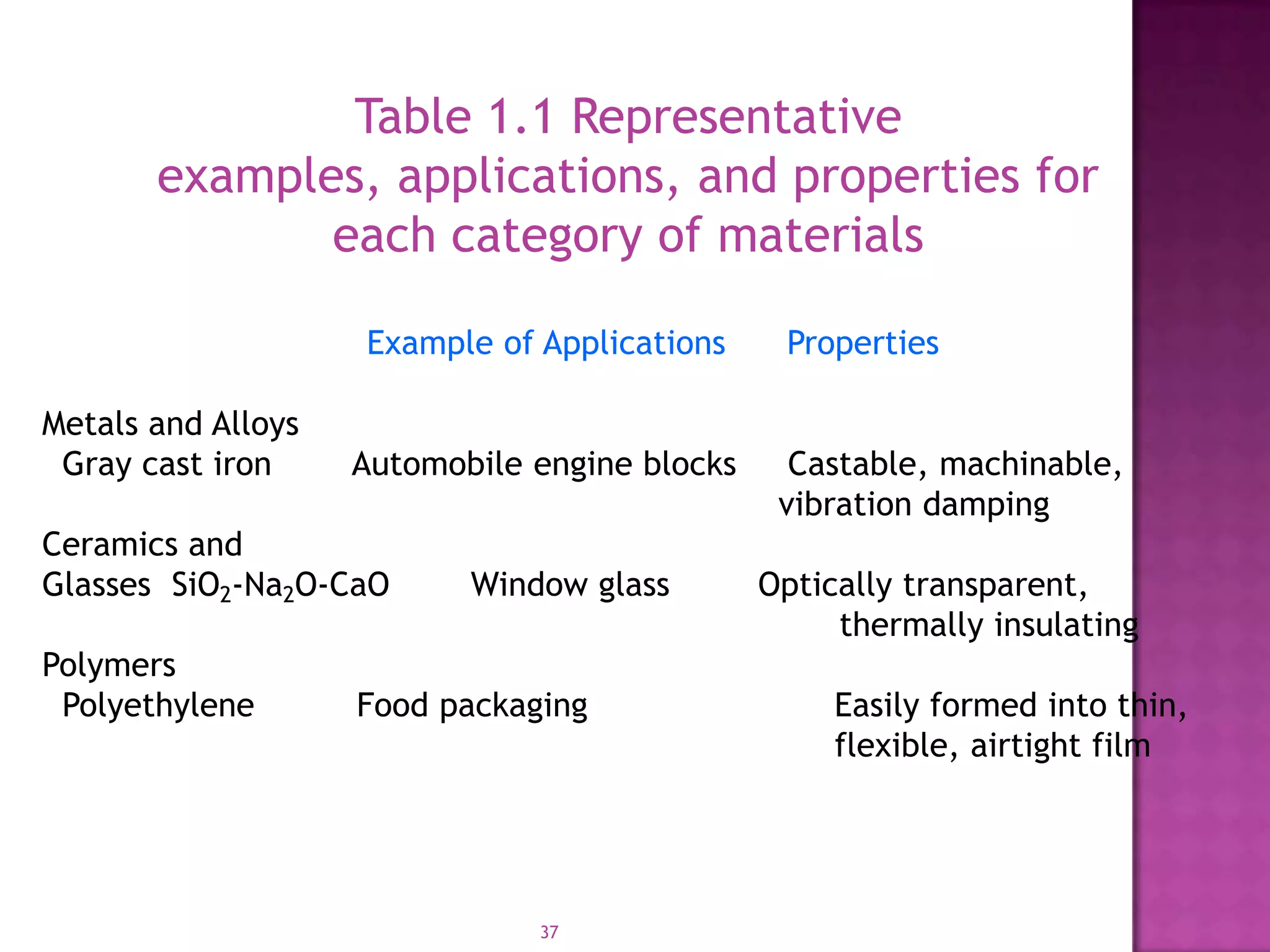
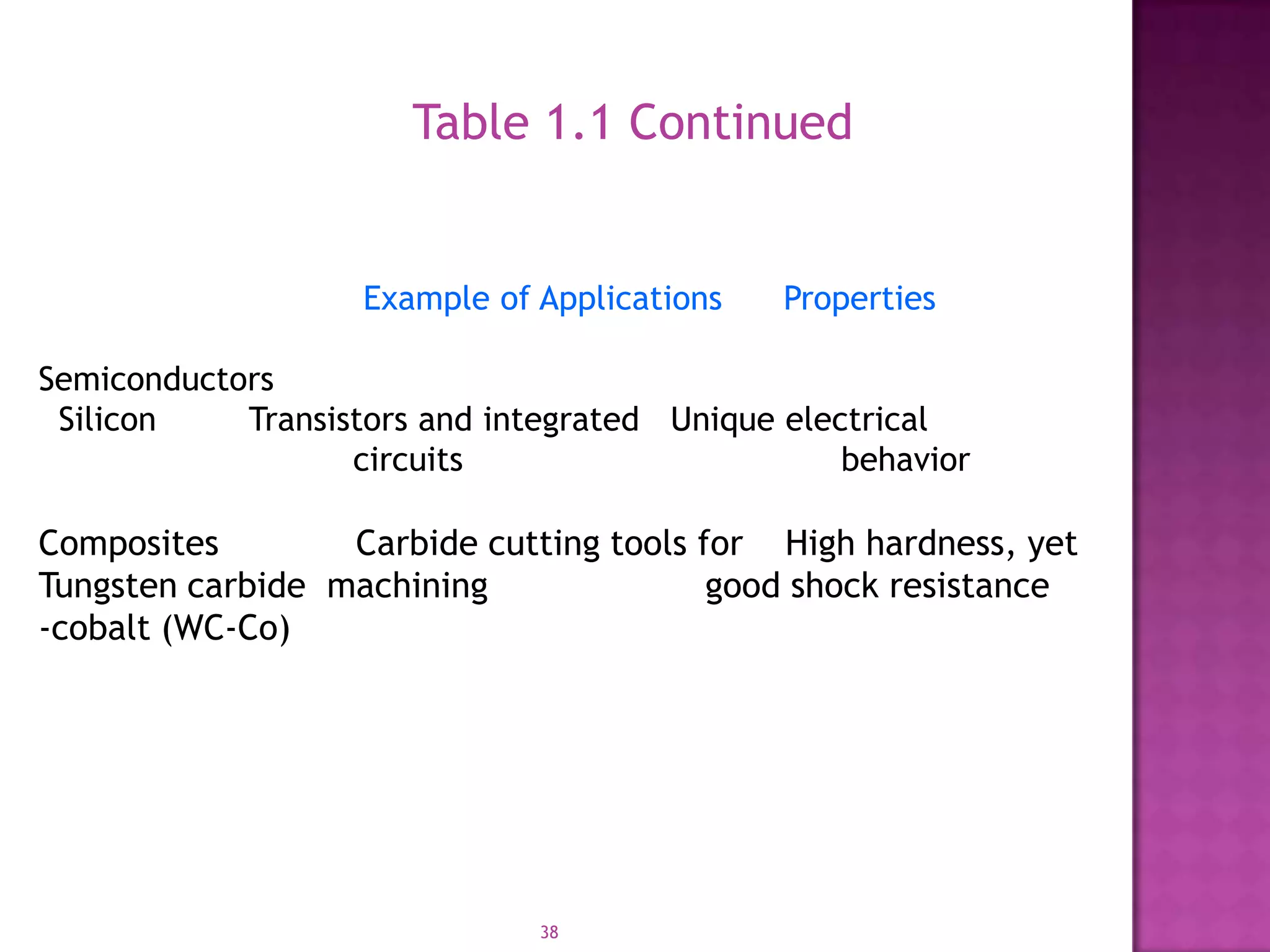
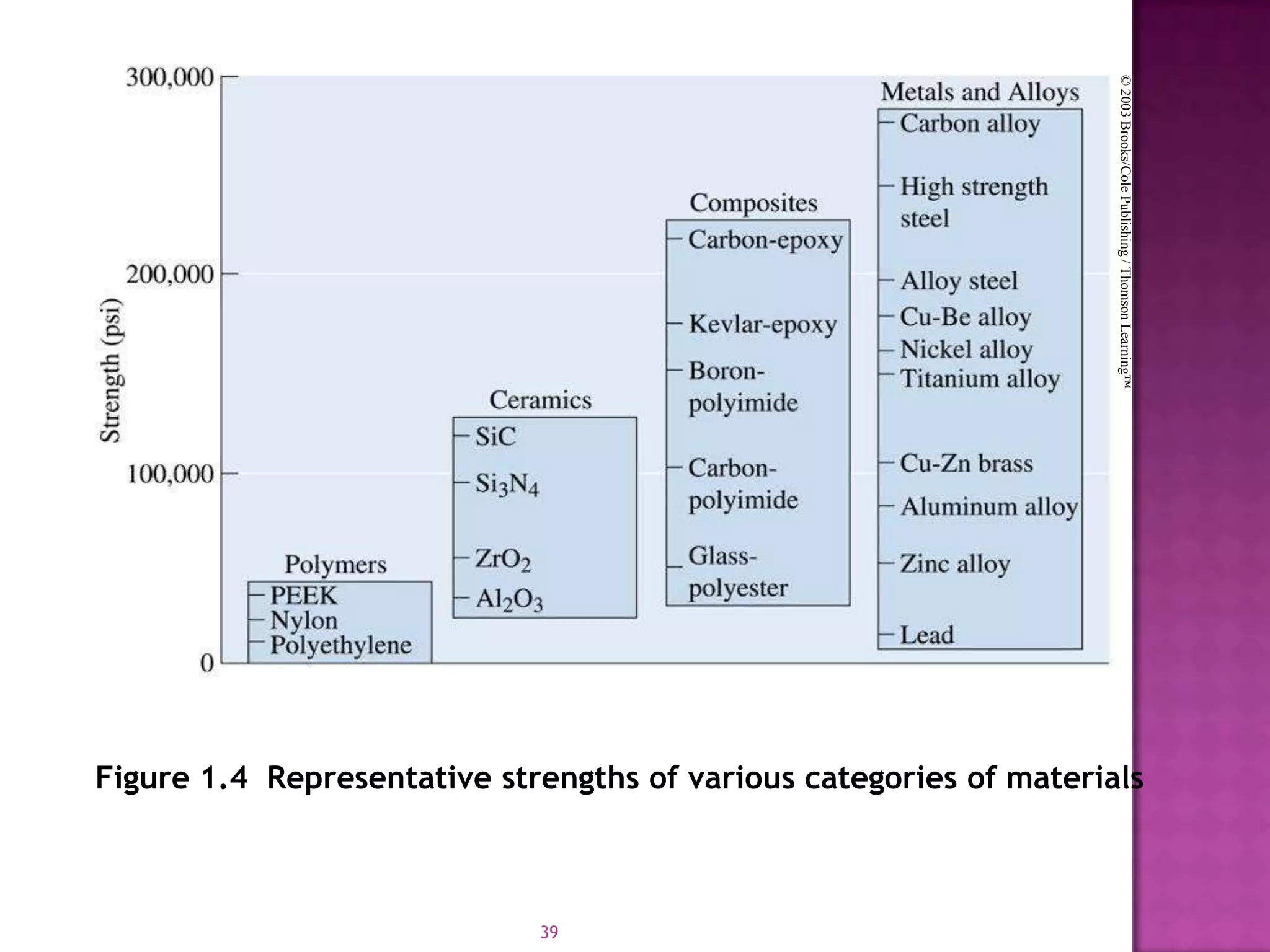
Material science and engineering is an interdisciplinary field that develops new materials and improves existing ones by understanding microstructure-composition-processing relationships. The field studies how a material's structure, synthesis, and processing affect its properties. Material scientists focus on underlying relationships between synthesis, processing, structure and properties, while material engineers translate materials into useful devices by controlling synthesis and processing to achieve desired structures and properties.