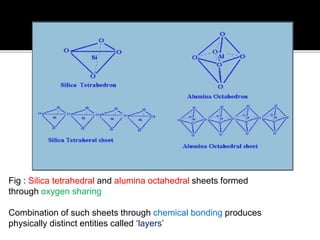
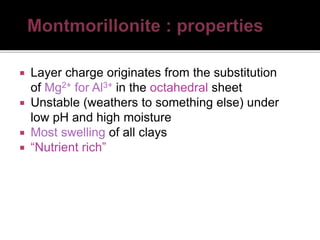
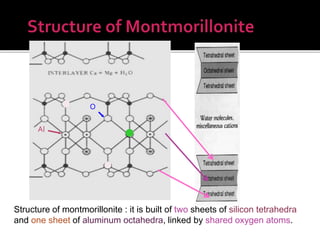
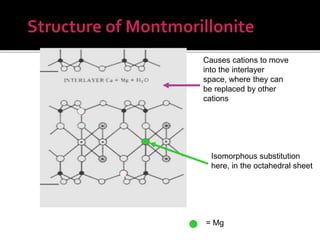
The document provides an in-depth overview of clay, specifically its geological composition, properties, and various applications in construction, manufacturing, and polymer chemistry. It details the processes of intercalation and exfoliation in modifying clay structures to create nano composites, highlighting the effects of different cations and organophilic modifications. Additionally, it discusses the advantages and uses of modified clays in industries such as oil removal, paint formulations, and as additives in drilling fluids.