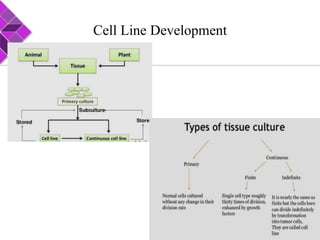
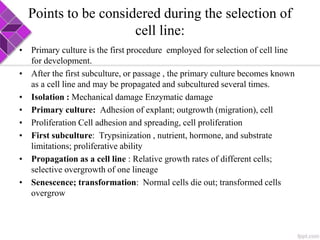
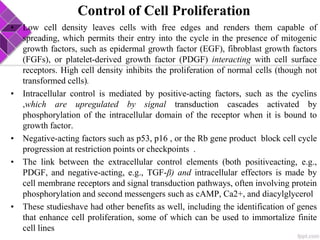
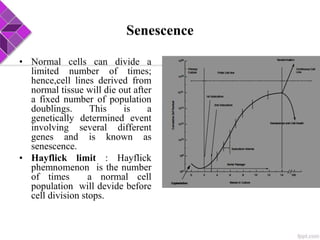
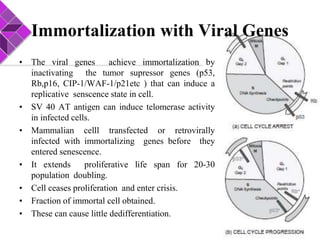
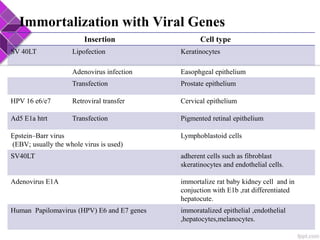
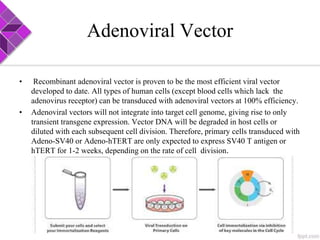
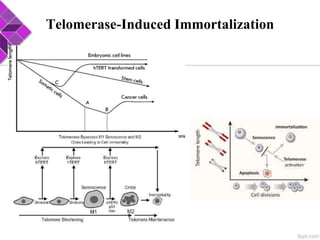
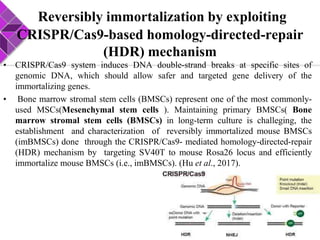
This document discusses various methods for selecting and developing cell lines for research. It covers primary culture isolation, subculture propagation to establish a cell line, control of cell proliferation through growth factors and intracellular regulators, senescence limits on cell divisions, differentiation inhibiting proliferation, and the need for continuous cell lines. Methods to immortalize cell lines include viral genes like SV40 LT and HPV E6/E7 to inactivate tumor suppressors, adenoviral and retroviral vectors, telomerase induction with hTERT, use of oncogenes, and cell hybridization. The goal is to generate stable, consistent cell lines that proliferate indefinitely while maintaining similar phenotype to the original tissue.