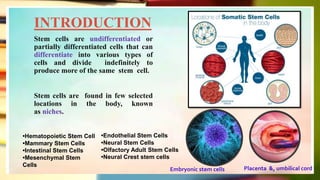
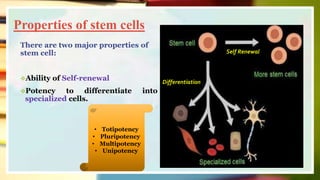
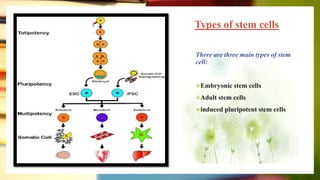
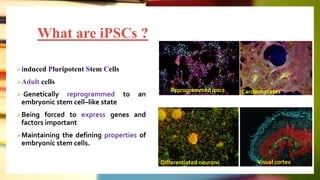
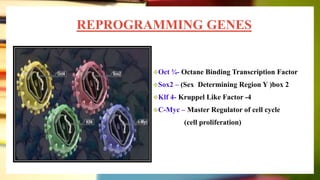
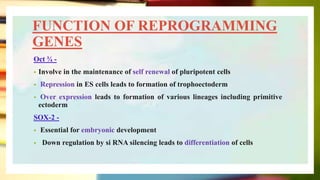
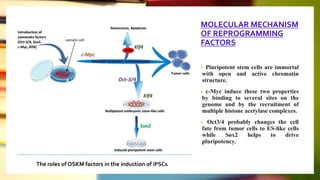
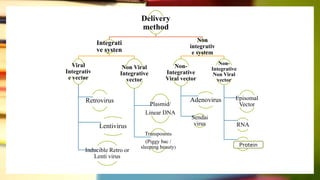
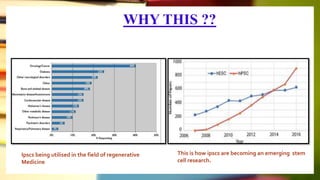
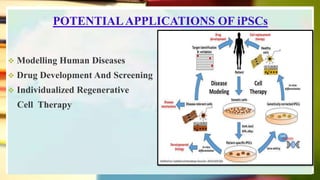
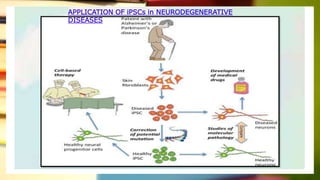
This document discusses induced pluripotent stem cells (iPSCs). iPSCs are adult cells that have been genetically reprogrammed to an embryonic stem cell-like state. This allows them to differentiate into various cell types. The document outlines the properties and characterization of iPSCs, the genes involved in reprogramming, potential applications for modeling diseases and regenerative medicine, and advantages and limitations of iPSCs compared to embryonic stem cells.