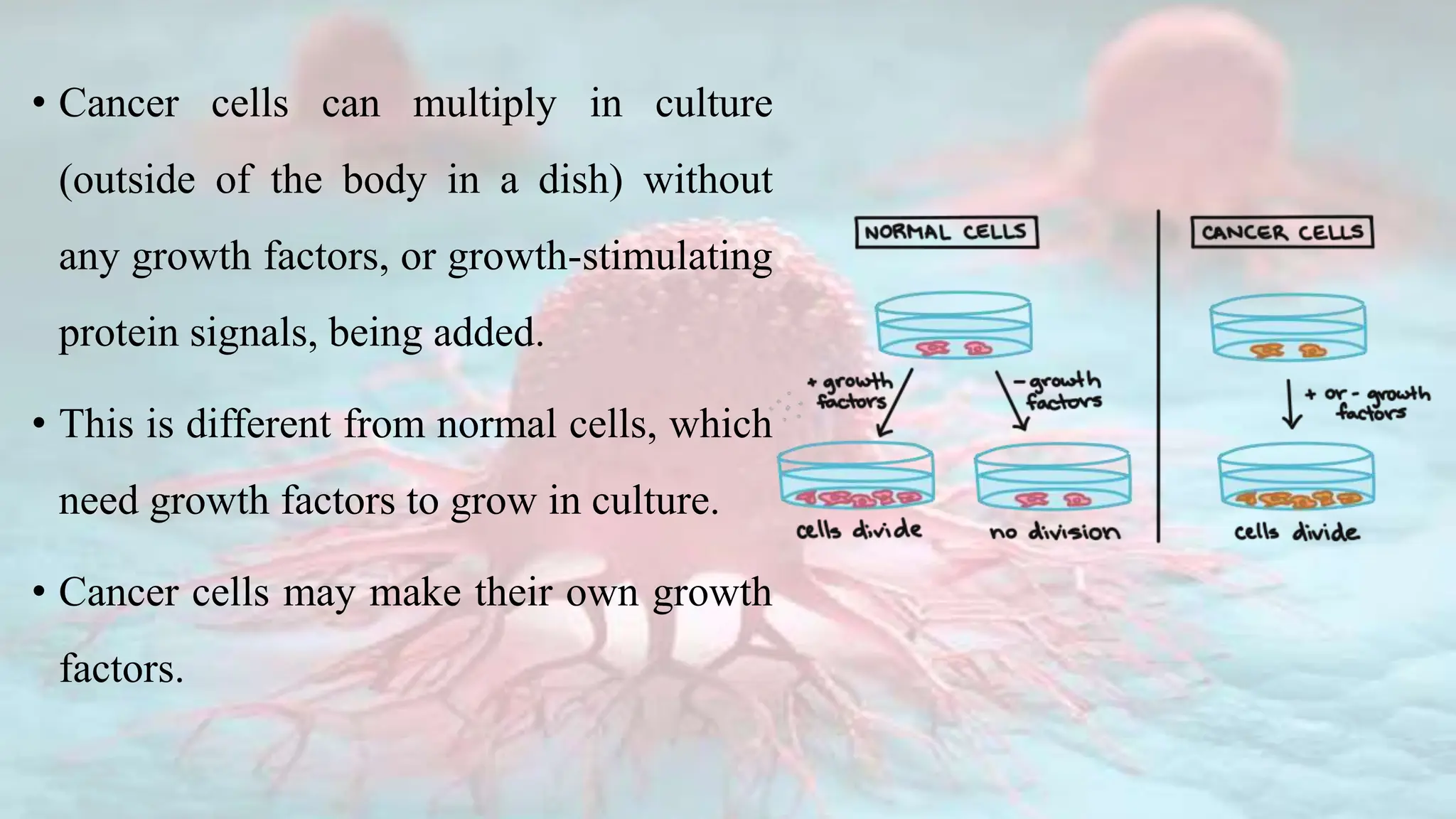
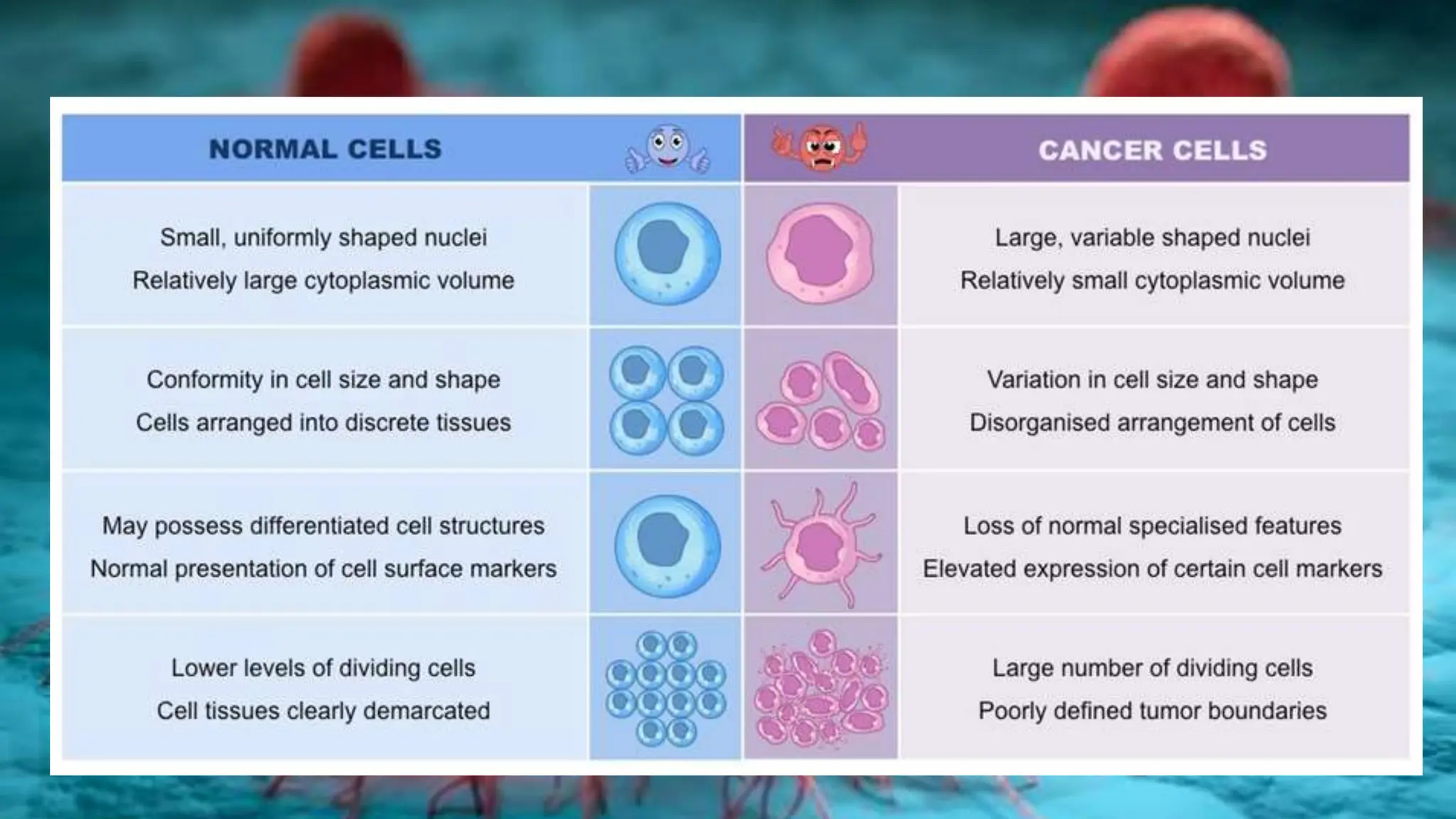
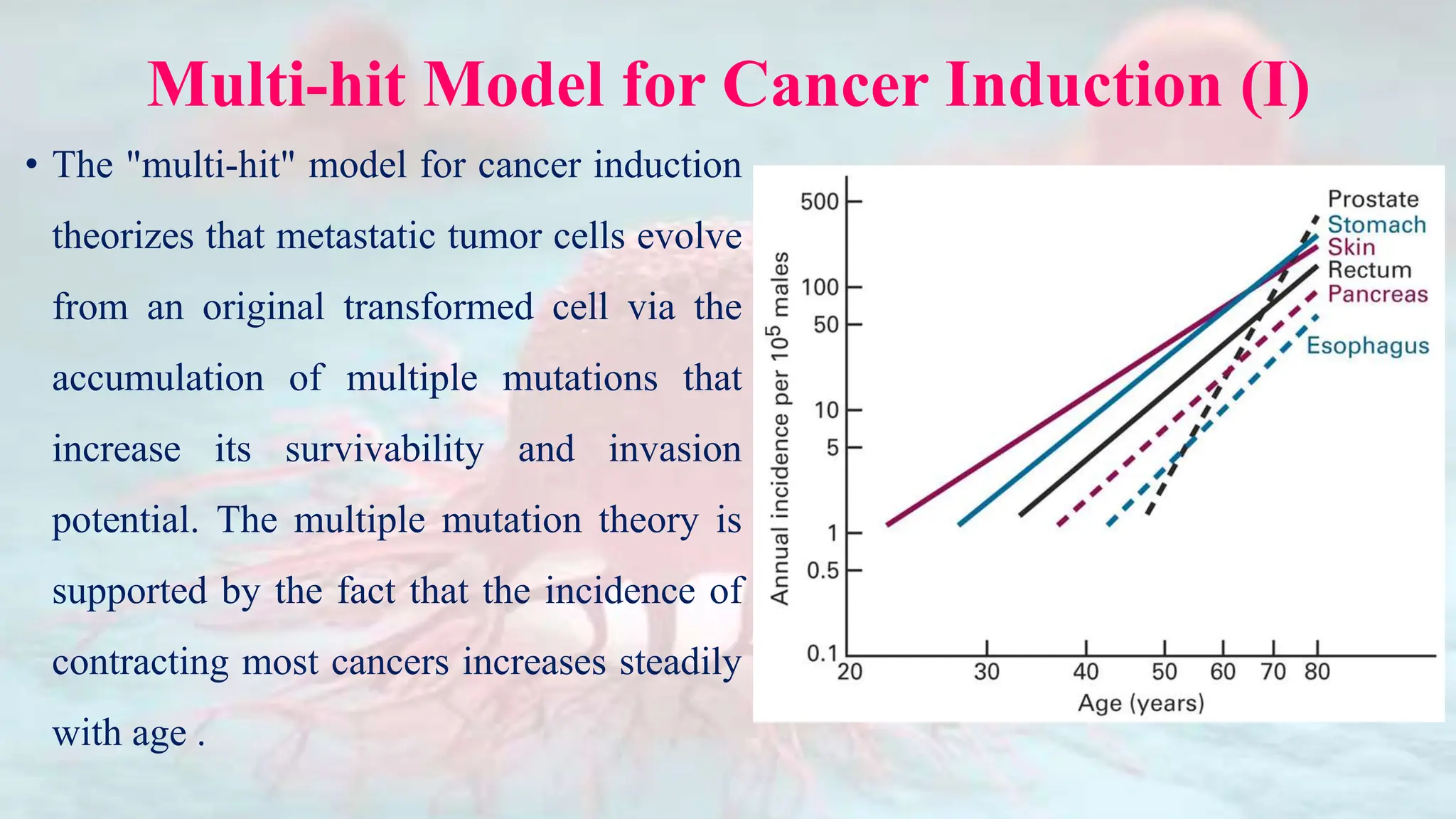
Cancer is characterized by the uncontrolled growth of abnormal cells, which can form tumors that may be benign or malignant. The development of cancer is influenced by genetic mutations, environmental factors, and the dysfunction of normal cellular processes, including cell division and apoptosis. Various types of cancer, such as carcinoma, sarcoma, leukemia, lymphoma, and central nervous system cancers, arise from specific tissues in the body, with specific mutations in proto-oncogenes and tumor suppressor genes contributing to their development.