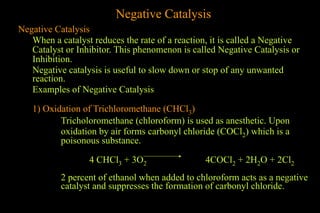
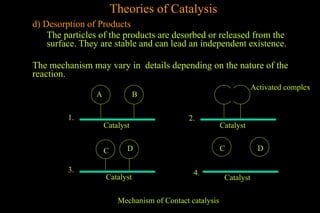
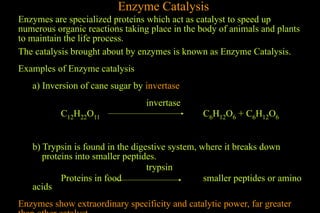
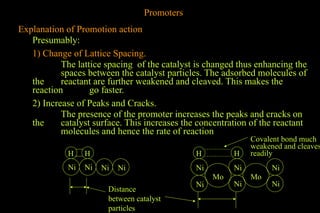
The document discusses various aspects of catalysis, including promoters that enhance catalyst activity, catalytic poisoning by substances that deactivate catalysts, and autocatalysis where reaction products act as catalysts. It also covers negative catalysis, which slows down reactions, and the theories of catalysis, including intermediate compound formation and adsorption theory. Additionally, it elaborates on acid-base catalysis and enzyme catalysis, highlighting the efficiency and specificity of enzymes in biological reactions.





![Autocatalysis
The reaction of oxygen binding to hemoglobin in a cooperative manner is given by the
schematic diagram:
The oxygen-hemoglobin dissociation curve provides a graphical
representation of the relationship between hemoglobin saturation and
partial pressure of oxygen, which exhibits cooperative binding behavior.
The equation for this curve can be represented as follows:
Y-axis: % Hemoglobin saturation
X-axis: Partial pressure of oxygen (PO2)
The curve can be described by the equation:
Y = [HbO2]
[HbO2] + [Hb]](https://image.slidesharecdn.com/lecture12nro-241228192801-b60cd1e5/85/Lecture-12_NROgyfftftftdtydtytyttdtydttt-7-320.jpg)