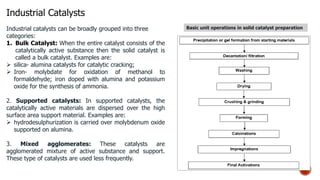
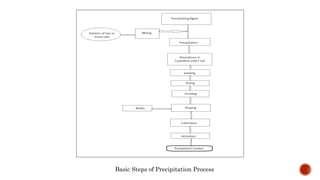
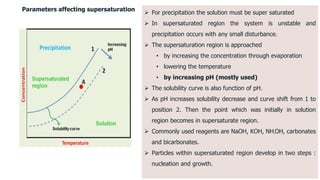
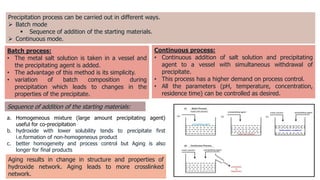
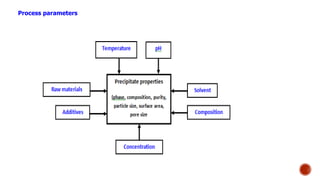
Bulk catalysts and supported catalysts can be prepared through various methods including precipitation, sol-gel, impregnation, and physical mixing. Precipitation involves supersaturating a solution and adding a precipitating agent to form solid precipitates. It is commonly used to produce bulk catalysts and support materials like alumina. Sol-gel forms a colloidal solution or sol that transitions to a gel network and then a solid oxide through condensation reactions. Impregnation involves depositing an active component onto a porous support by submerging the support in a metal salt solution. Supported catalysts are often prepared through impregnation.