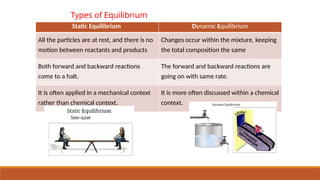
This document covers the topic of equilibrium in chemistry for Class XI, explaining reversible reactions, types of equilibrium (static and dynamic), and their applications in physical and chemical processes. It details how equilibrium is achieved in systems such as solid-liquid, liquid-vapor, and gas-liquid, and discusses the law of mass action, equilibrium equations, and constants. The document also highlights the dynamic nature of chemical equilibrium and the differences between homogeneous and heterogeneous equilibria.
















![1) For solid liquid equilibrium, there is only one temperature (melting point) at 1
atm (1.013 bar) at which the two phases can coexist. Melting point is fixed at
constant pressure
2) For liquid vapour equilibrium, the vapour pressure is constant at a given
temperature.
3) For dissolution of solids in liquids, [Solute(s) Solute (solution)] the solubility is
constant at a given temperature.
4) For dissolution of gases in liquids, [Gas(g) Gas (aq)] the concentration of a gas
in liquid is proportional to the pressure (concentration) of the gas over the
liquid. [gas(aq)]/[gas(g)] is constant at a given temperature
Summary of Equilibrium In Physical
Processes](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-19-320.jpg)




![Law Of Mass Action
• Proposed by Guldberg and Waage so also known as Guldberg - Waage Law
• Rate of a chemical reaction is directly proportional to the product of the
concentrations of the reactants
Rate of forward reaction,
Rate of reverse reaction,
At equilibrium,
In the early days of
chemistry,
concentration was
called “active mass”.
[A], [B], [C] and [D] are the
equilibrium concentrations
of the reactants and
products](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-28-320.jpg)
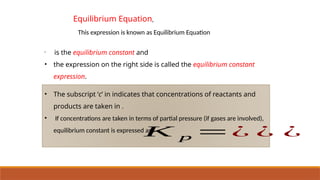
![Equilibrium Law or Law of Chemical Equilibrium
• At a given temperature, the product of concentrations of the reaction products
raised to the respective stoichiometric coefficient in the balanced chemical
equation divided by the product of concentrations of the reactants raised to their
individual stoichiometric coefficients has a constant value. This is known as the
Equilibrium Law or Law of Chemical Equilibrium.
• Equilibrium constant for the reaction
K c =
[ C ]c
[ D ]d
[ A ]
a
[ B ]
b
• While writing expression for equilibrium
constant, symbol for phases (s, l, g, aq) are
generally ignored.](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-30-320.jpg)

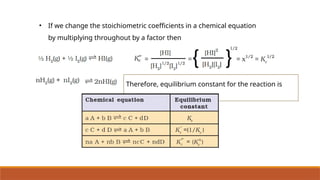
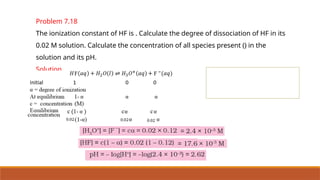
![Problem 7.1
The following concentrations were obtained for the formation of NH3 from N2 and
H2 at equilibrium at 500K.
[N2] = 1.5 × 10–2
M [H2] = 3.0 ×10–2
M and [NH3] = 1.2 ×10–2
M. Calculate
equilibrium constant.
Solution:-](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-33-320.jpg)



![HETEROGENEOUS EQUILIBRIA
• Equilibrium in a system having more than one phase is called heterogeneous
equilibrium.
• Heterogeneous equilibria often involve pure solids or liquids.
• Molar concentration of a pure solid or liquid is constant (i.e., independent of
the amount present).
• In other words if a substance ‘X’ is involved, then [X(s)] and [X(l)] are constant,
whatever the amount of ‘X’ is taken.
• While [X(g)] and [X(aq)] will vary as the amount of X in a given volume varies.](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-37-320.jpg)
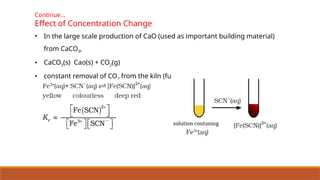
![HETEROGENEOUS EQUILIBRIA
• thermal dissociation of calcium carbonate
• On the basis of the stoichiometric equation, we can write,
• Since [CaCO3(s)] and [CaO(s)] are both constant,
For the existence of heterogeneous
equilibrium pure solids or liquids must
also be present at equilibrium, but their
concentrations or partial pressures do not
appear in the expression of the
equilibrium constant.](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-38-320.jpg)
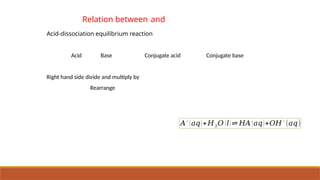
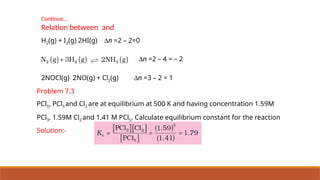
![Relation between and
aA+bB⇌cC+dD
K c =
[ C ]c
[ D ]d
[ A ]
a
[ B ]
b K p =¿¿ ¿
pV = nRT
where Dn = (number of moles of gaseous products) – (number of moles of gaseous
reactants) in the balanced chemical equation.](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-40-320.jpg)




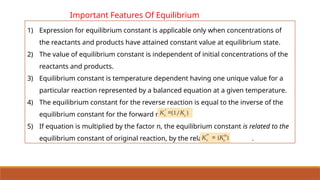

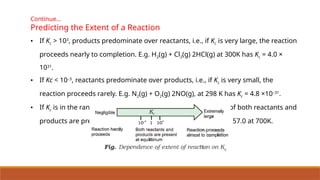

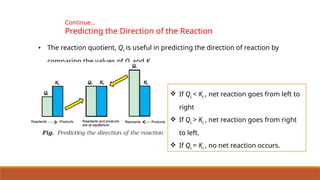
![Problem 7.7
The value of Kc for the reaction 2A B + C is 2 × 10–3
. At a given time,
the composition of reaction mixture is [A] = [B] = [C] = 3 × 10–4
M. In
which
direction the reaction will proceed?
Solution:- For the reaction the reaction quotient Qc is given by,
as [A] = [B] = [C] = 3 × 10–4
M
as Qc > Kc so the reaction will proceed in the reverse direction.](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-51-320.jpg)
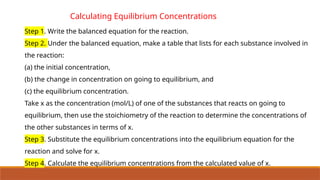
![Problem 7.9
3.00 mol of PCl5 kept in 1L closed reaction vessel was allowed to attain
equilibrium at 380K. Calculate composition of the mixture at equilibrium. Kc=
1.80
Solution:-
Step 1: PCl5 PCl3 + Cl2
Step 2: Initial concentration : 3.0 0 0
Let x mol per litre of PCl5 be dissociated
At equilibrium: (3 – x) x x
Step 3:
x =1.59
Step 4:
x2
+ 1.8x – 5.4 = 0
x = [–1.8 ± Ö(1.8)2
– 4(–5.4)]/2
x = [–1.8 ± Ö3.24 + 21.6]/2
x = [–1.8 ± 4.98]/2
x = [–1.8 + 4.98]/2 = 1.59](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-53-320.jpg)








![• The equilibrium can be shifted in the opposite direction
by adding reagents that remove Fe3+
or SCNions.
• For example, oxalic acid (H2C2O4), reacts with Fe3+
ions to
form the stable complex ion [Fe(C2O4)3]3–
, thus decreasing
the concentration of free Fe3+
(aq).
• So [Fe(SCN)]2+
dissociates to give Fe3+
• Because the concentration of [Fe(SCN)]2+
decreases, the
intensity of red colour decreases.
Continue…
Effect of Concentration Change](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-65-320.jpg)
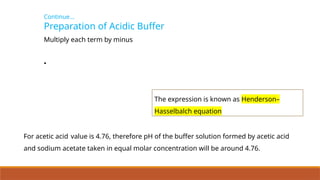
![Continue…
Effect of Concentration Change
• Addition of aq. HgCl2 also decreases red colour
because Hg2+
reacts with SCNions to form stable
complex ion [Hg(SCN)4]2–
.
• Removal of free SCN(aq) shifts the equilibrium from
right to left to replenish SCNions.
• Addition of potassium thiocyanate increases the
colour intensity of the solution as it shift the
equilibrium to right.](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-66-320.jpg)



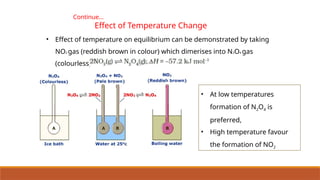
![Continue…
Effect of Temperature Change
• Effect of temperature can also be seen in an endothermic
reaction,
• At room temperature, the equilibrium mixture is blue due to
[CoCl4]2–
.
• When cooled in a freezing mixture, the colour of the mixture
turns pink due to [Co(H2O)6]3+
.](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-74-320.jpg)






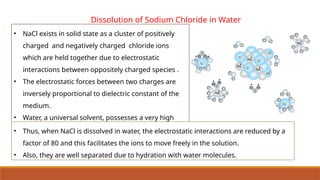
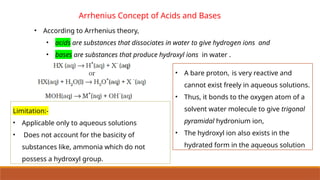

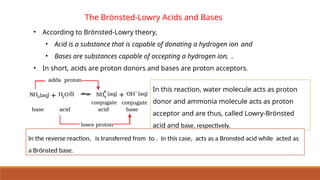
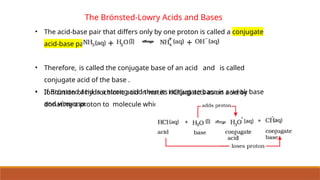
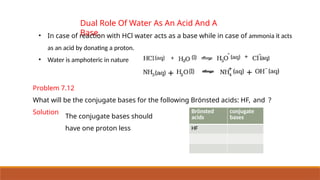
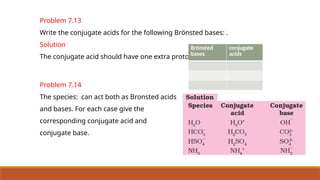




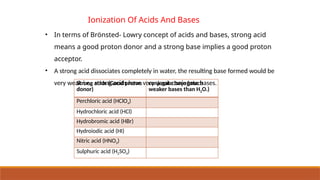


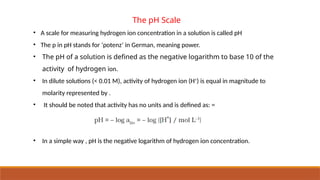
![Ionic Product Of Water
• Water is acting both as an acid and a base.
• Self ionization of water:-In pure water, one H2O molecule donates proton and acts as an
acid and another water molecules accepts a proton and acts as a base at the same time.
• The concentration of water is
omitted from the denominator
as water is a pure liquid and its
concentration remains
constant.
• [H2O] is incorporated within the
equilibrium constant to give a
new constant, , which is called
the ionic product of water.](https://image.slidesharecdn.com/class11chapter7equilibrium-241008043005-2c066c04/85/Class-11-Chapter-7-Equilibrium-pptx-presentation-96-320.jpg)