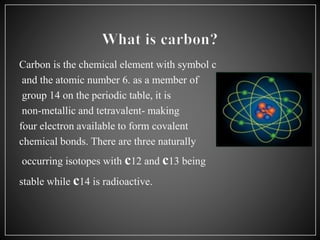
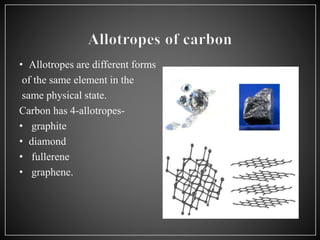
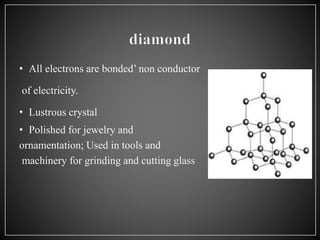
Carbon has 4 main allotropes - graphite, diamond, fullerene, and graphene. It is the chemical element with atomic number 6 and is a nonmetal. Carbon is widely found in nature and living organisms. Its allotropes have varying properties - diamond is the hardest material, graphite is an electrical conductor, fullerenes are spherical structures, and graphene is a strong two-dimensional material.