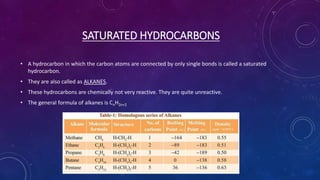
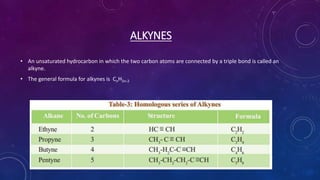
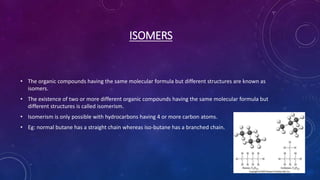
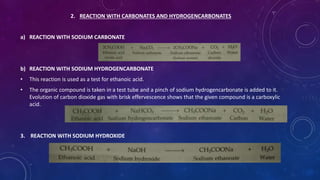
This document details the properties, allotropes, and compounds of carbon, highlighting its essential role in organic chemistry and daily life. It explains the unique features of carbon, including catenation and tetravalency, and describes different carbon allotropes such as diamond, graphite, and buckminsterfullerene. Additionally, it covers organic compounds, hydrocarbons, functional groups, combustion, and reactions involving carbon compounds, emphasizing their significance and applications.