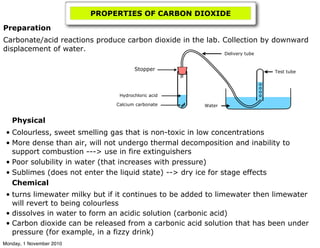
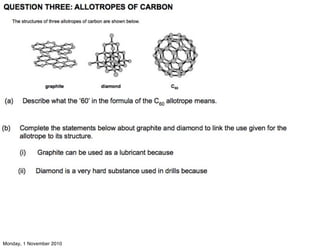
The document provides instructions for laboratory experiments and activities related to carbon chemistry and organic chemistry. In carbon chemistry, students will make models of carbon allotropes, describe the greenhouse effect, prepare carbon dioxide in the laboratory, and describe the carbon cycle. In organic chemistry, students will name and draw alkanes, explain trends in alkane melting and boiling points, describe fractional distillation of alkanes, and write combustion equations. Additional experiments include drawing and naming alkenes and writing polymerization equations.