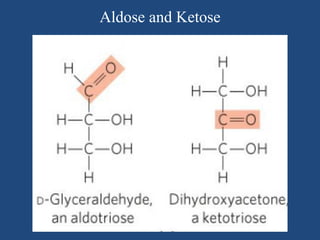
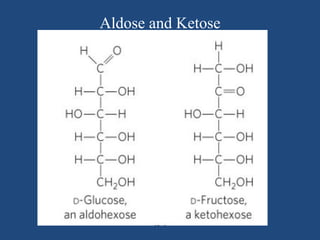
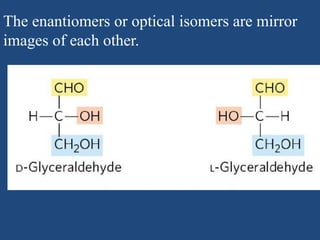
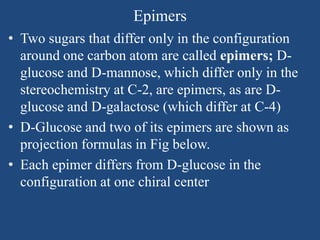
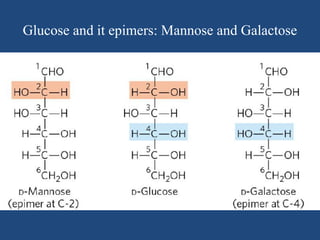
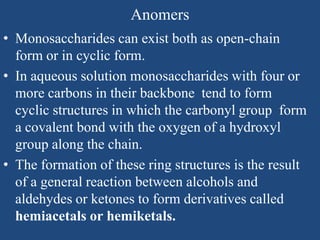
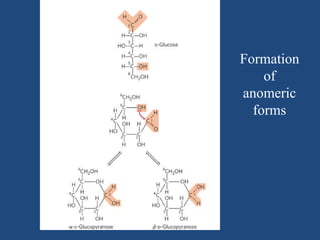
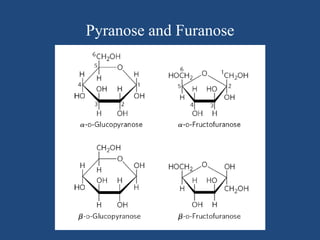
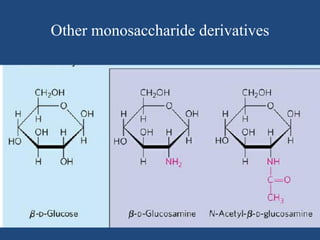
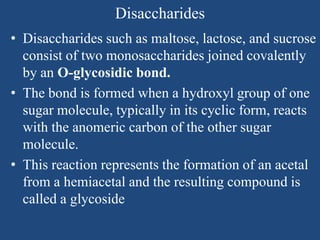
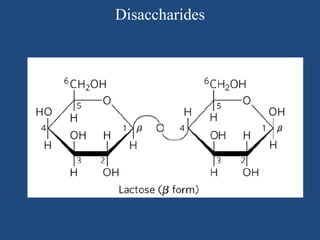
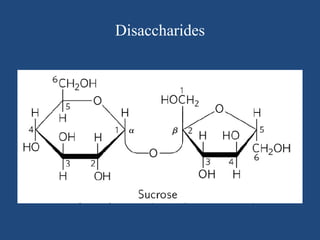
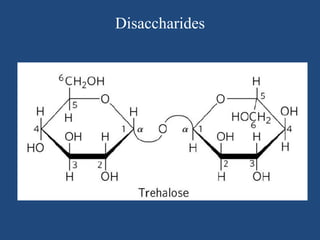
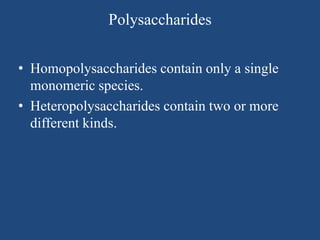
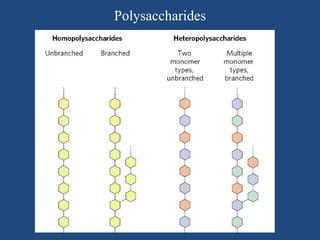
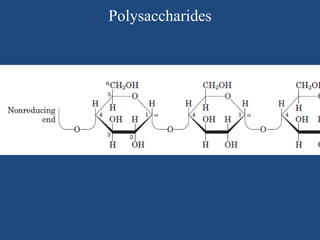
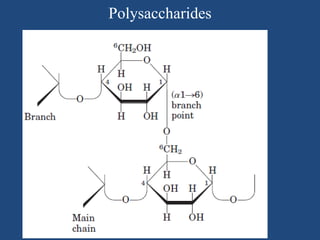
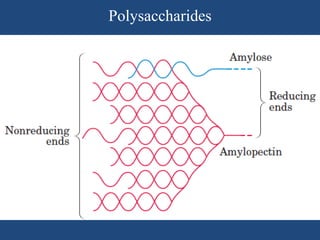
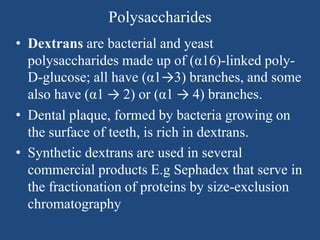
The document provides an in-depth overview of carbohydrates, detailing their structure, classification, and functions. It categorizes carbohydrates into monosaccharides, oligosaccharides, and polysaccharides, explaining their chemical properties, bonding mechanisms, and biological roles. Key examples such as glucose, sucrose, and cellulose are discussed, highlighting their significance in nutrition and cellular structure.