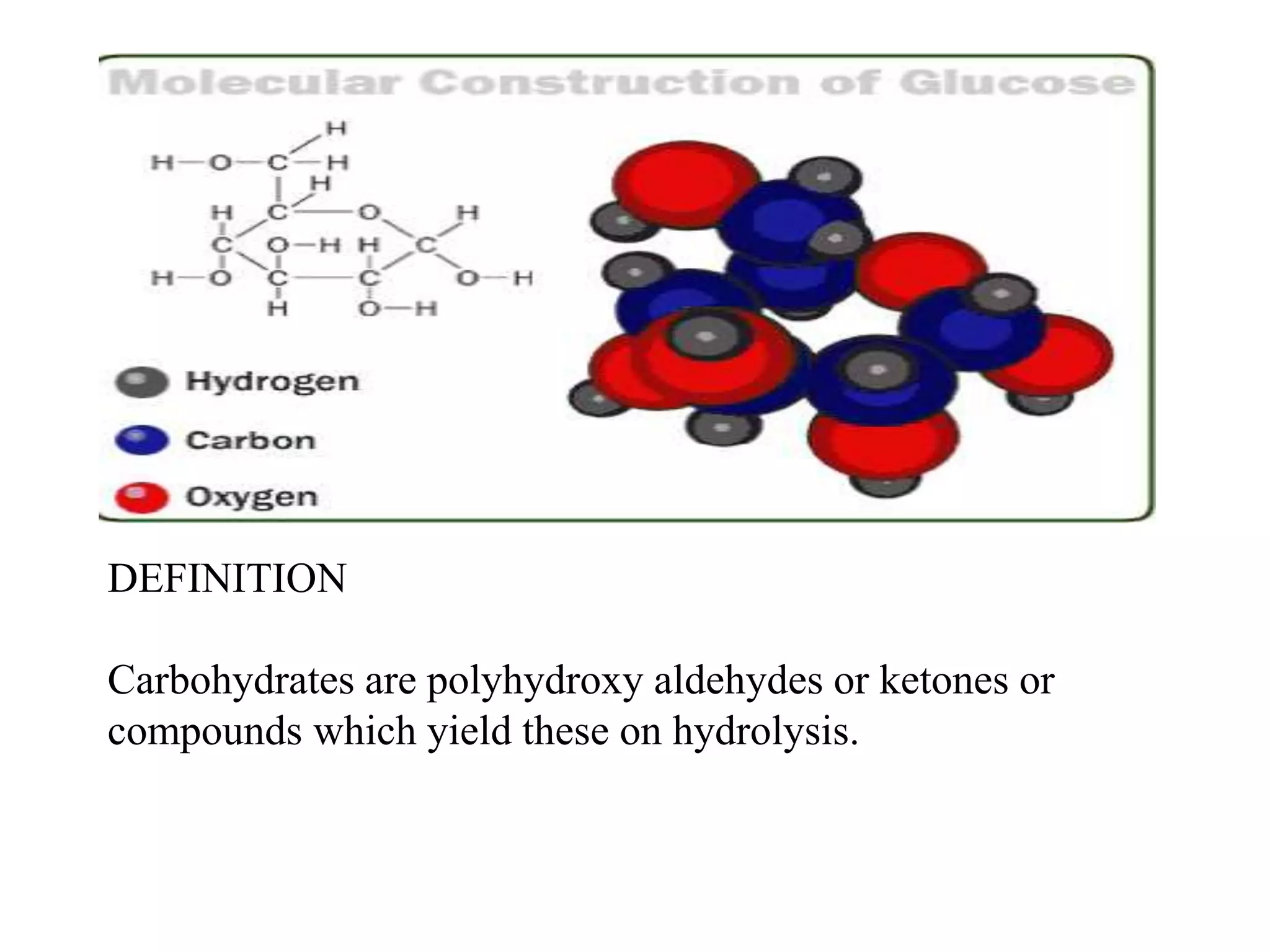
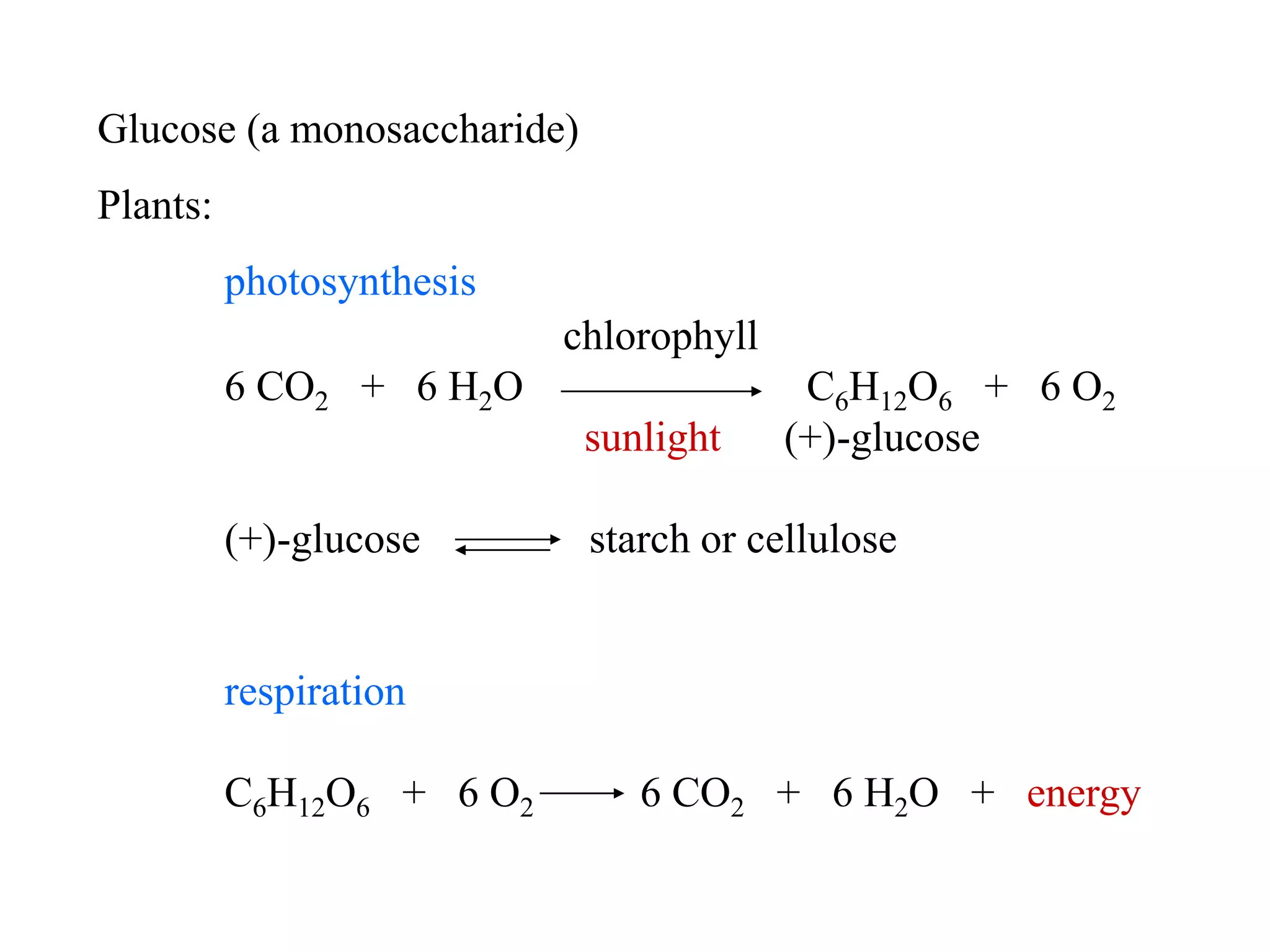
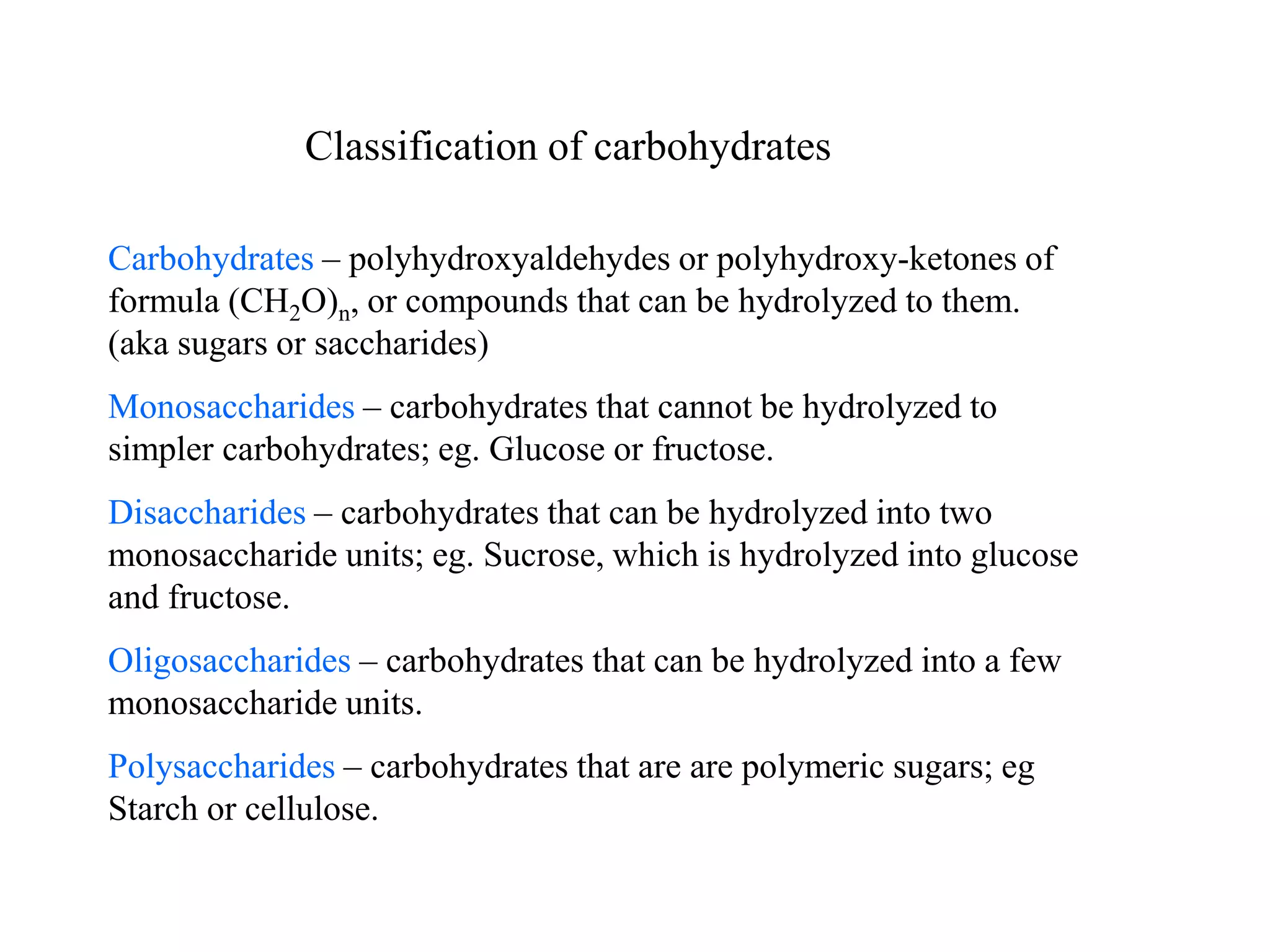
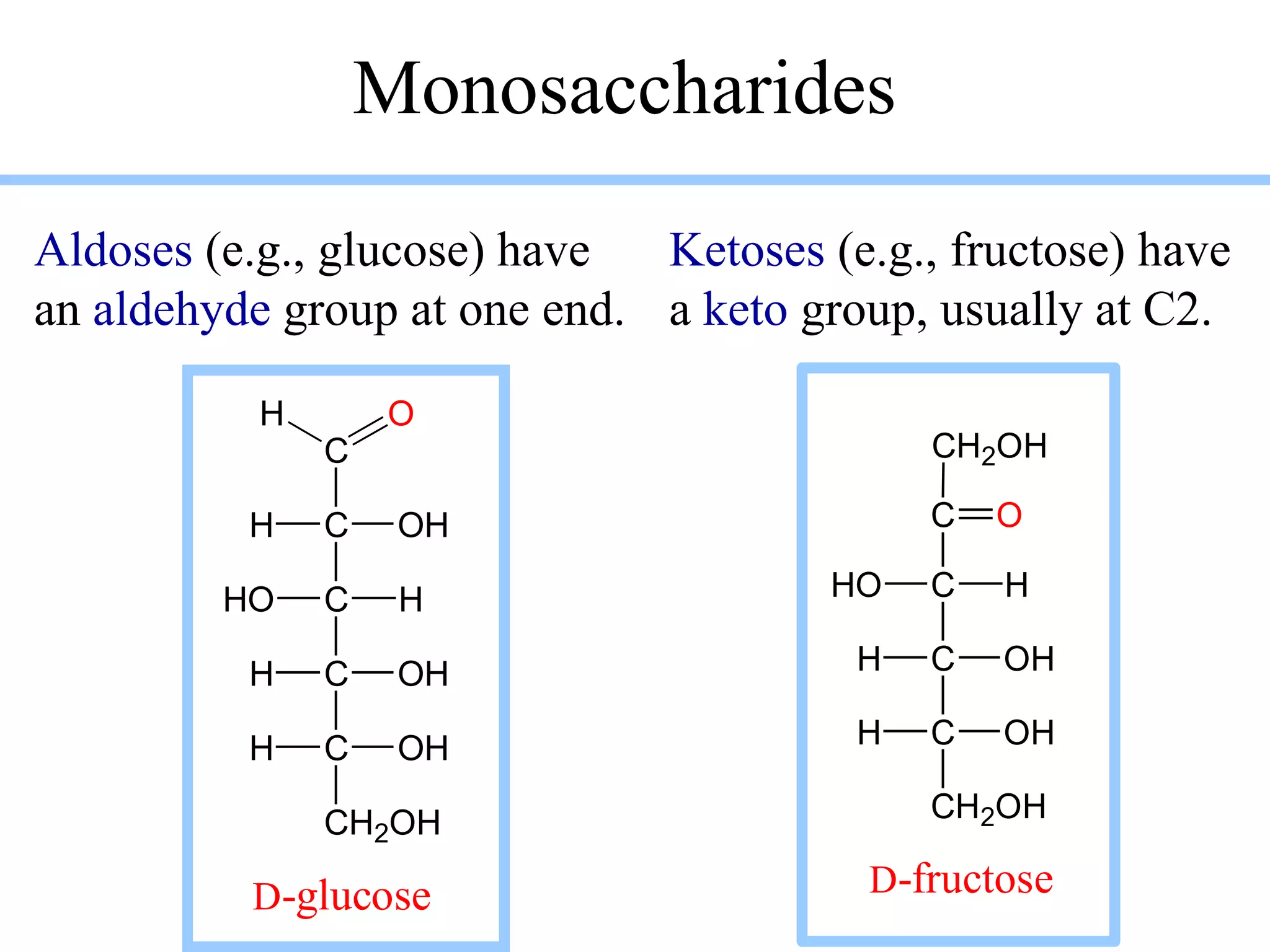
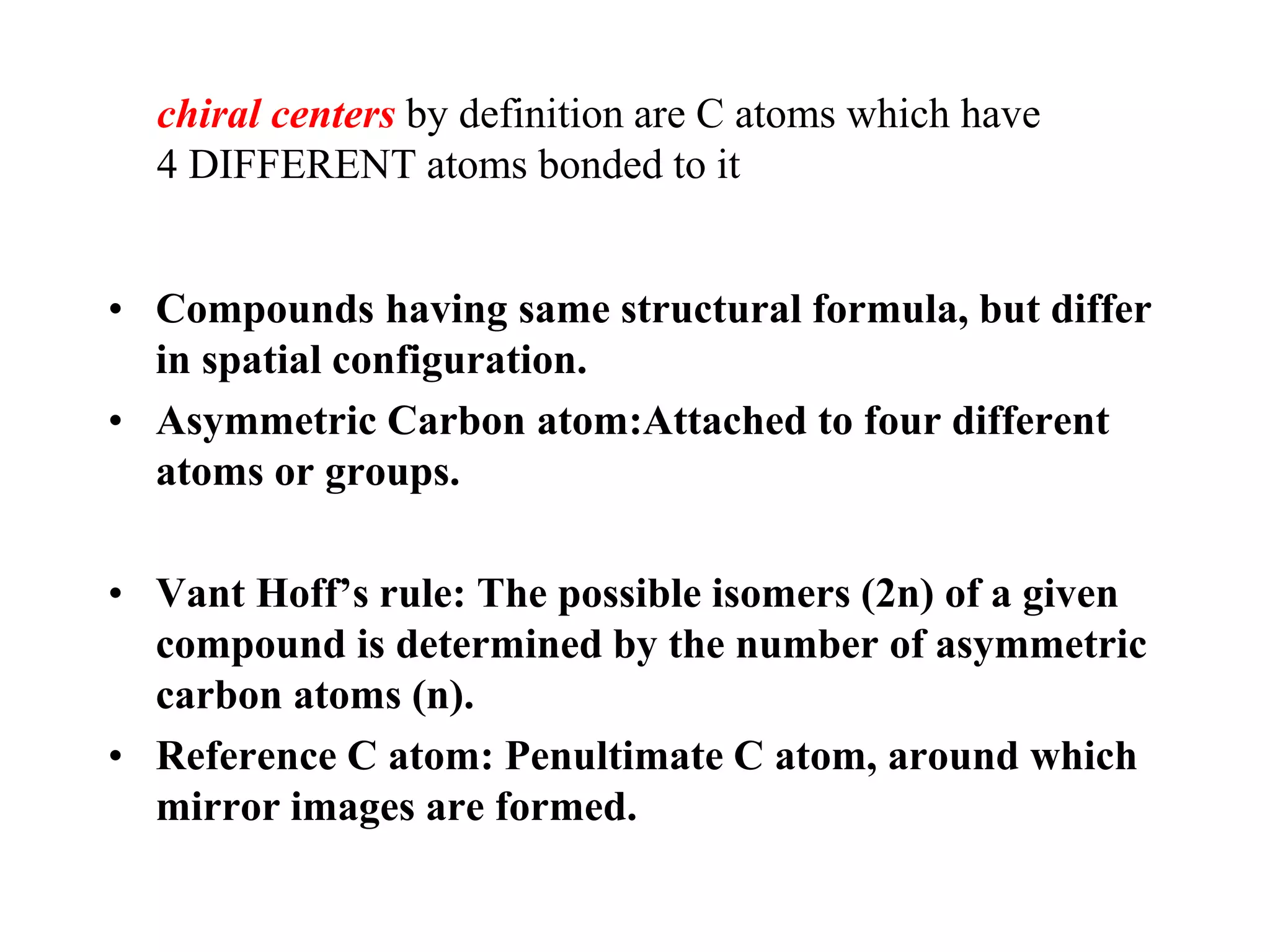
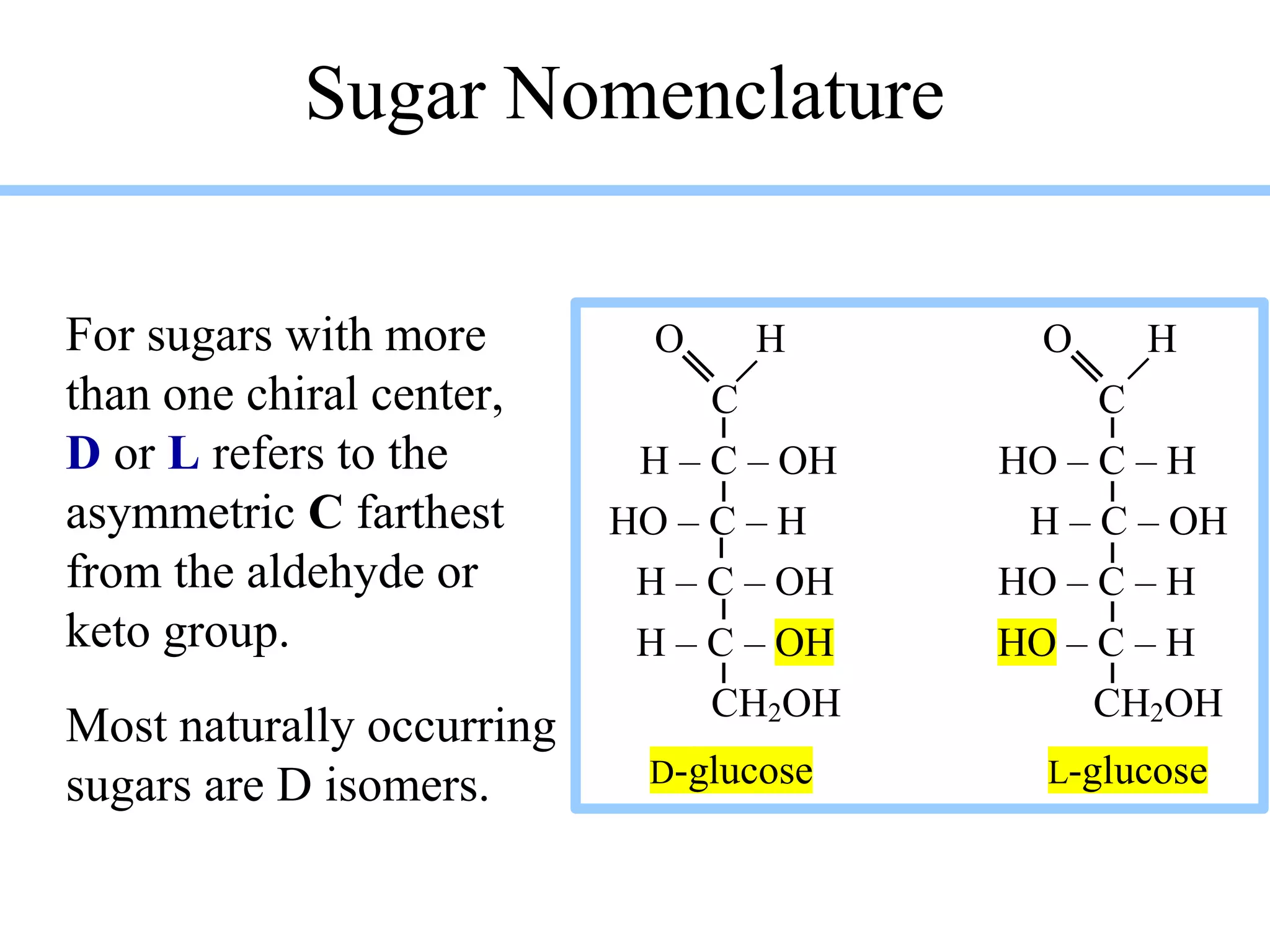
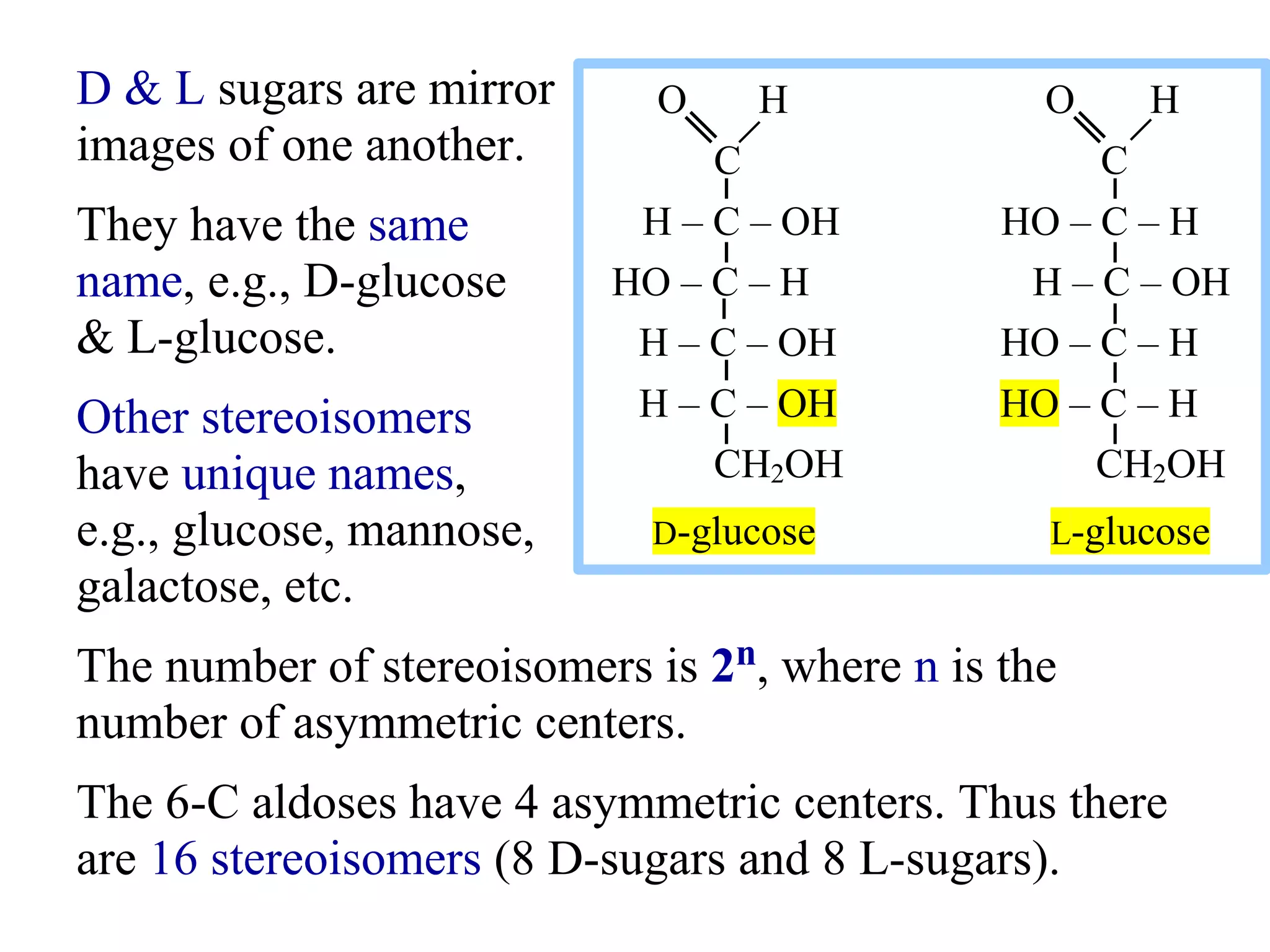
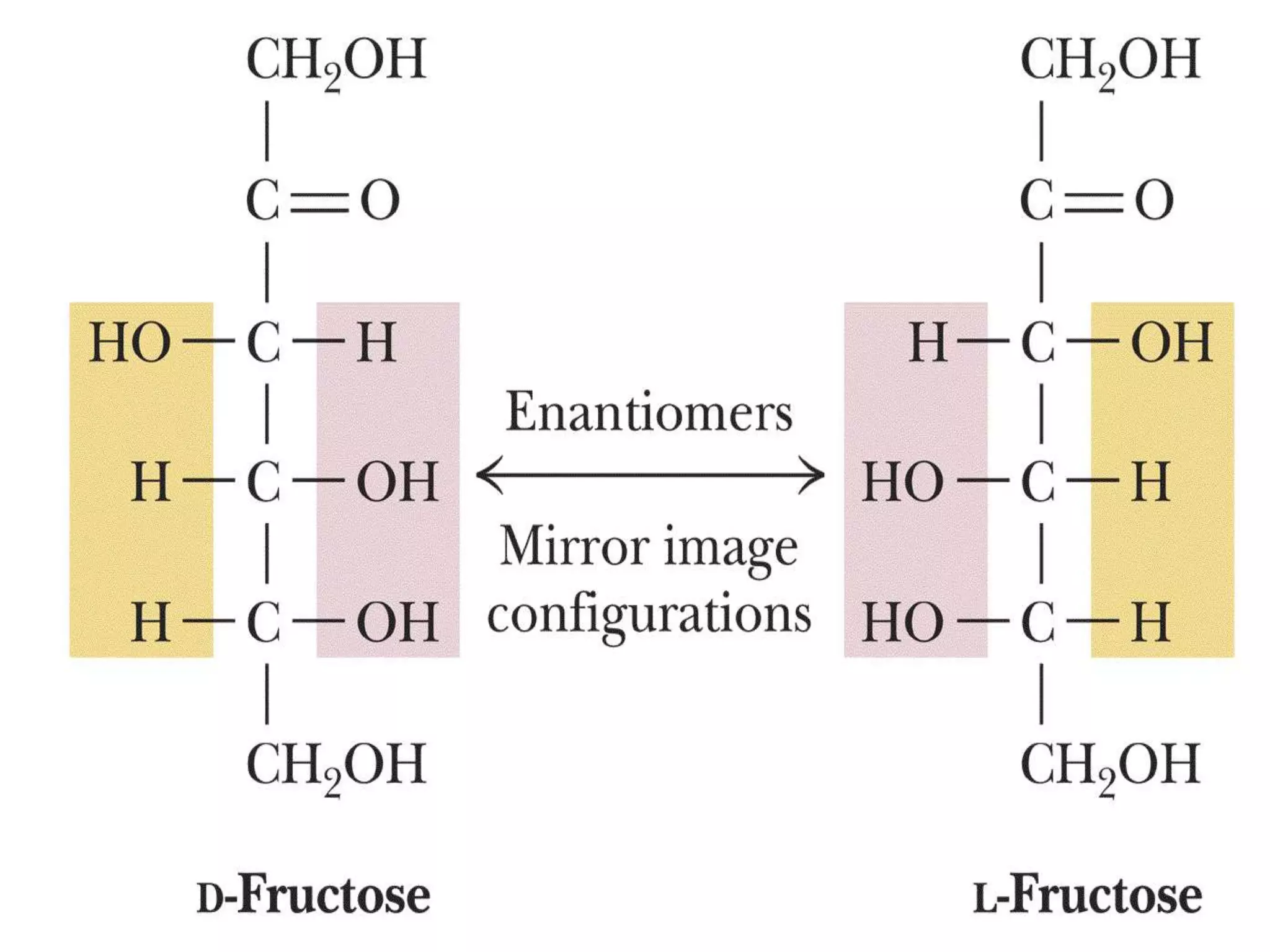
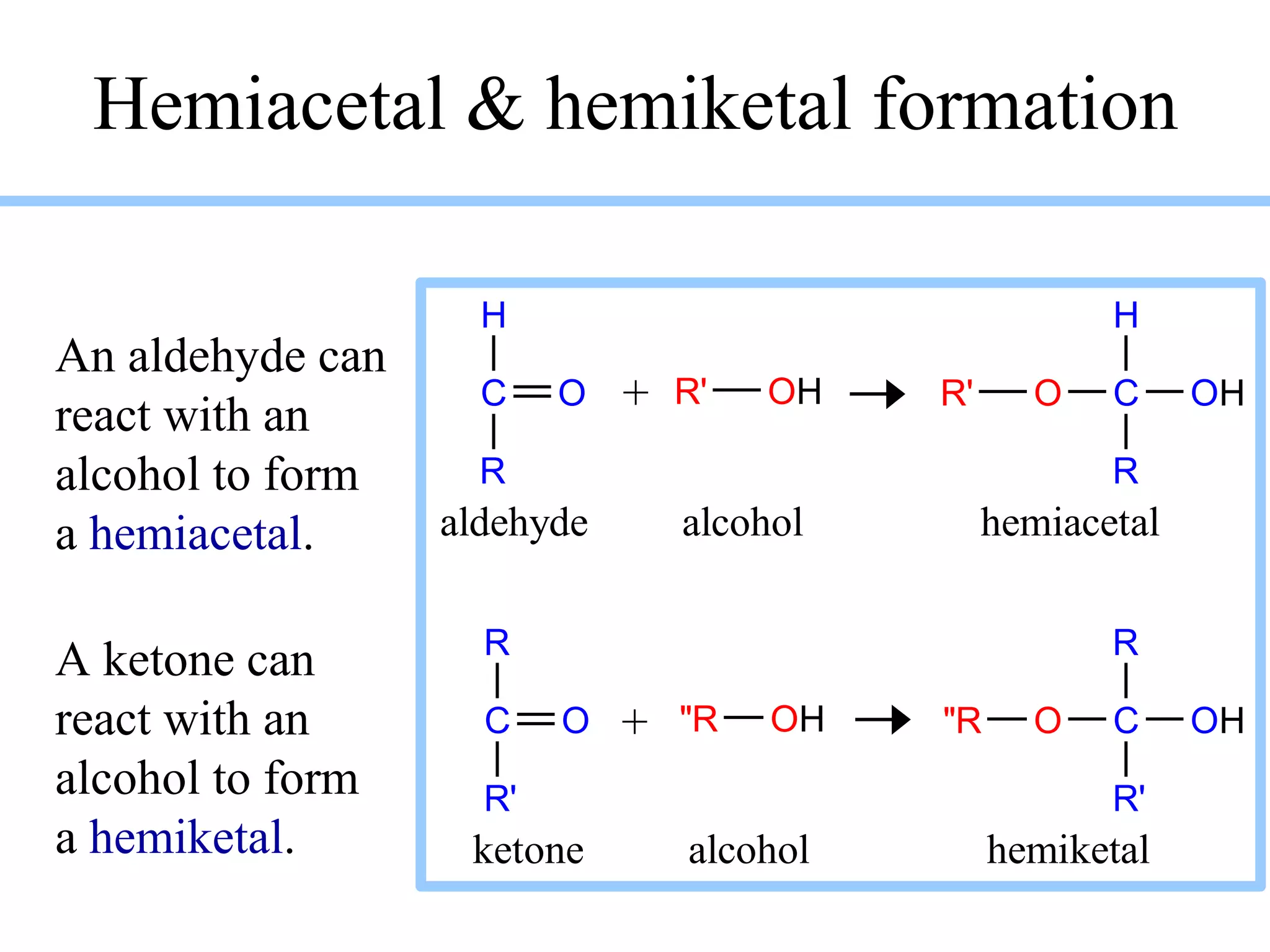
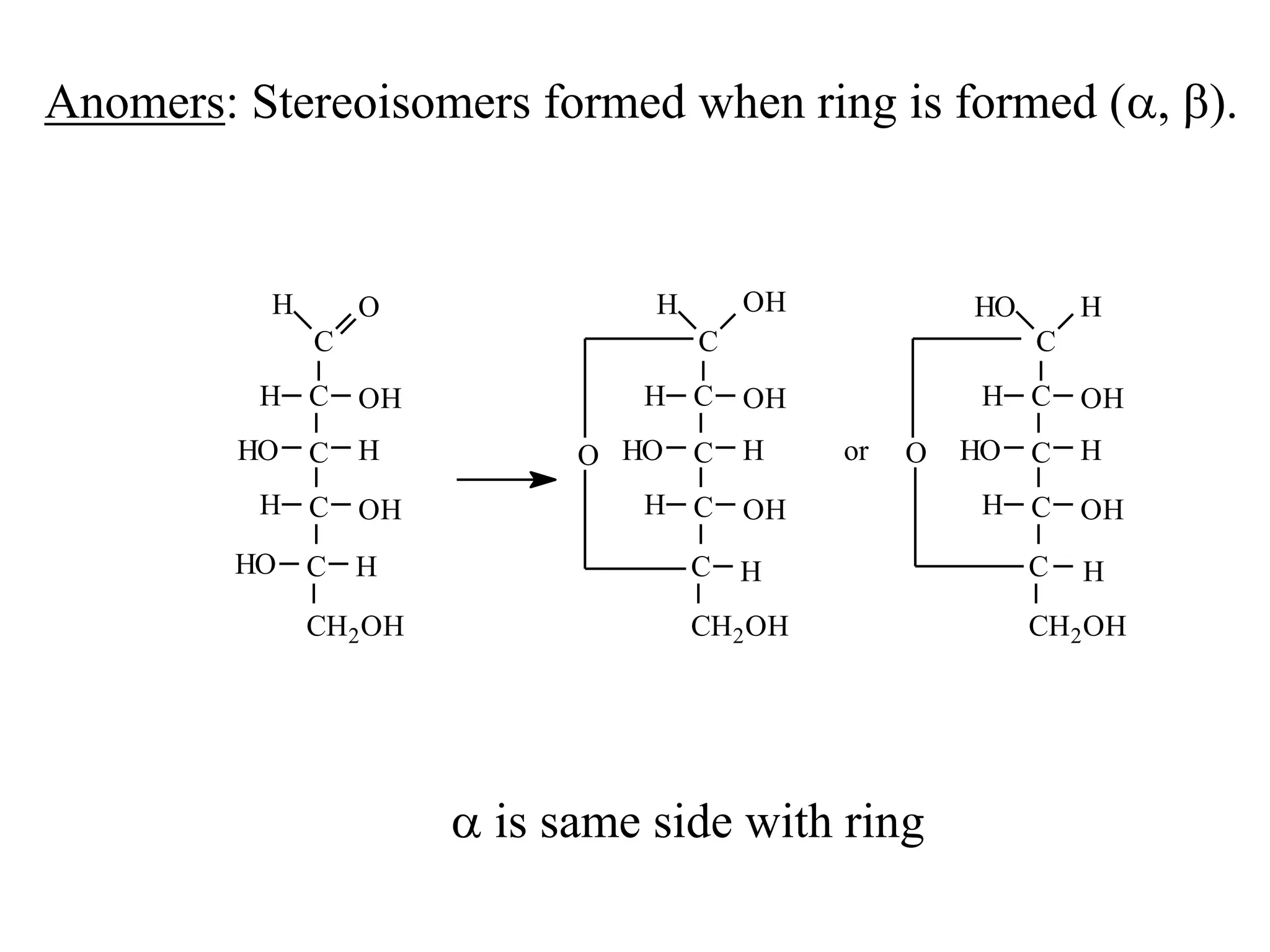
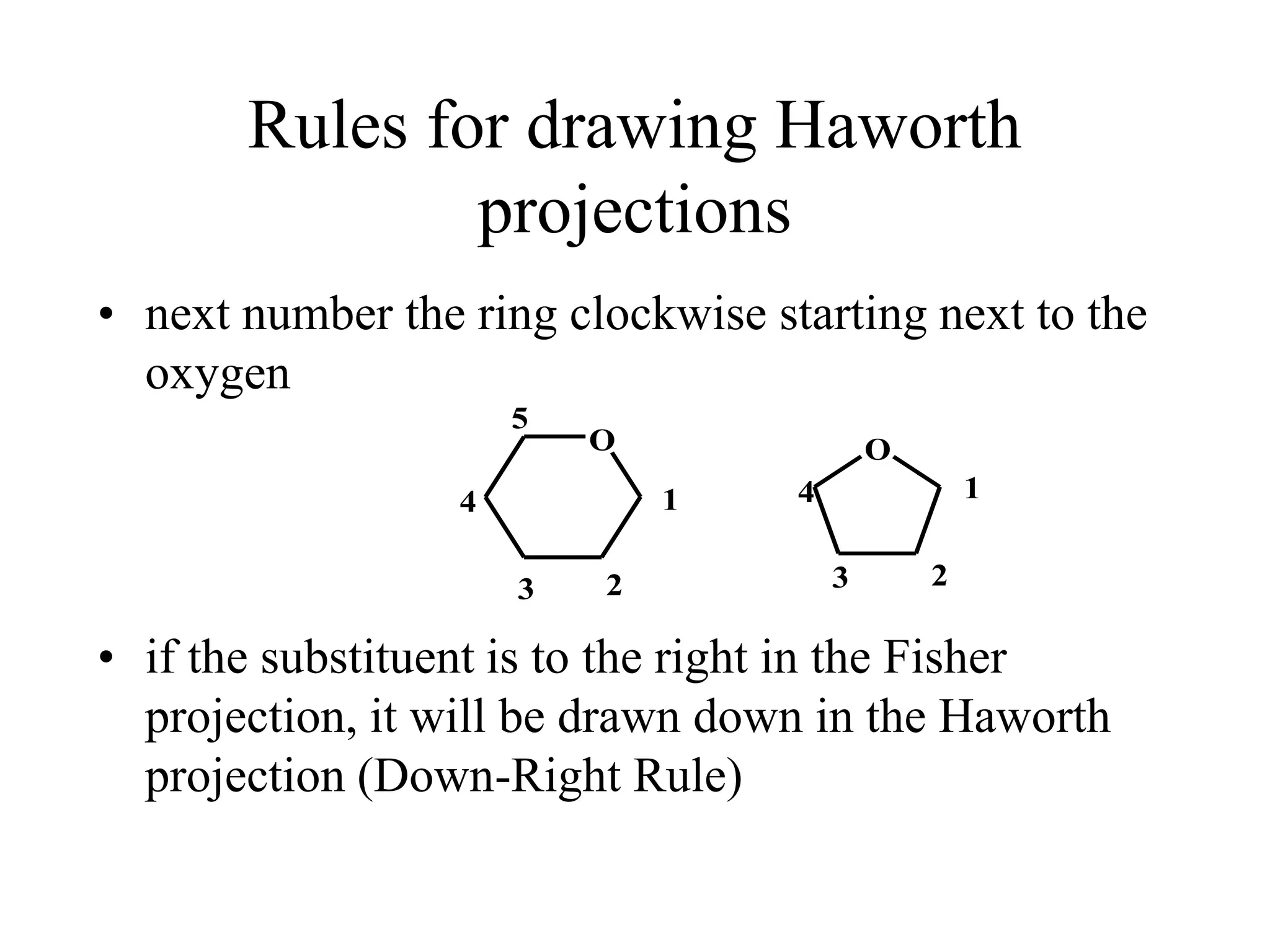
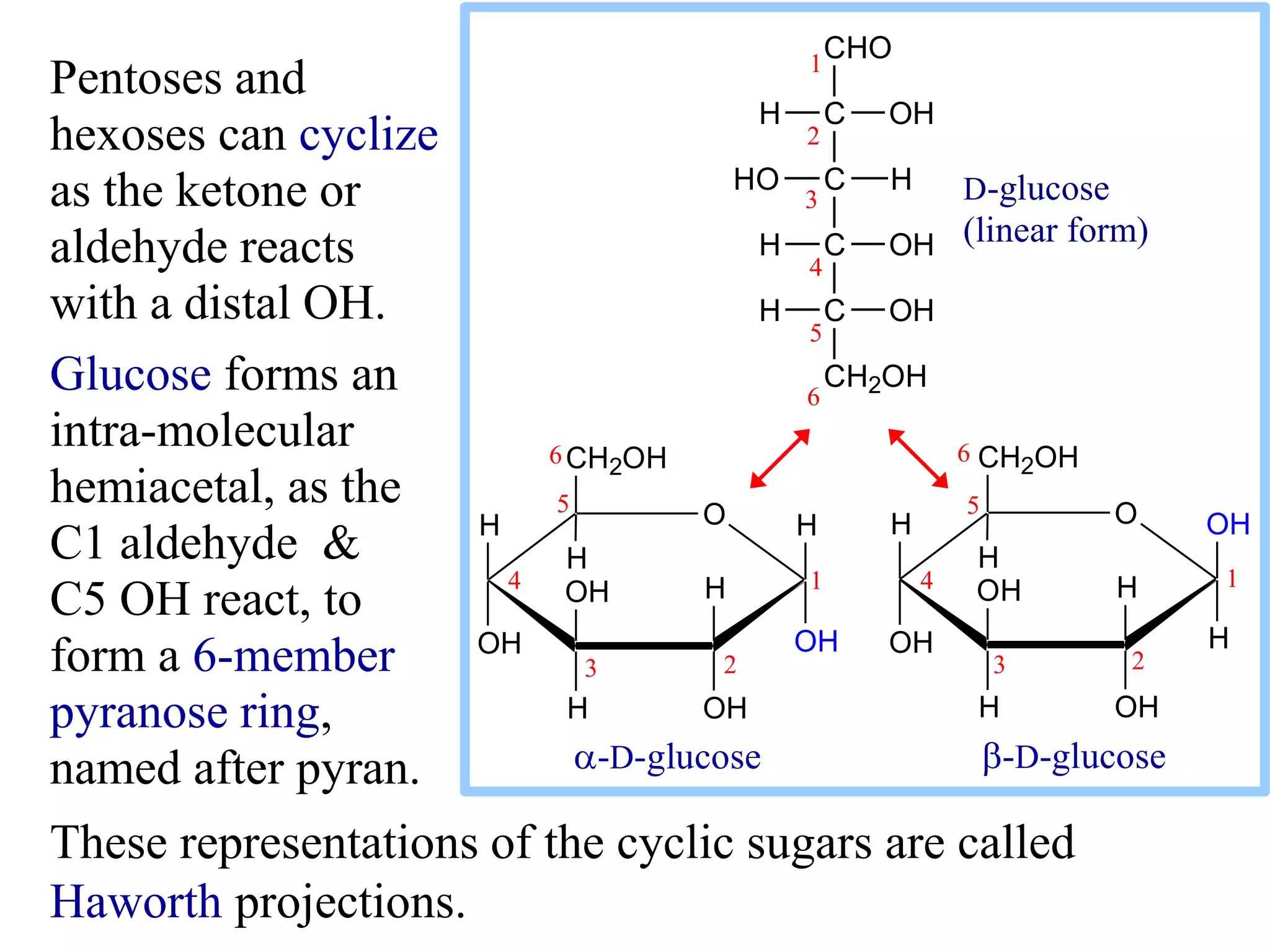
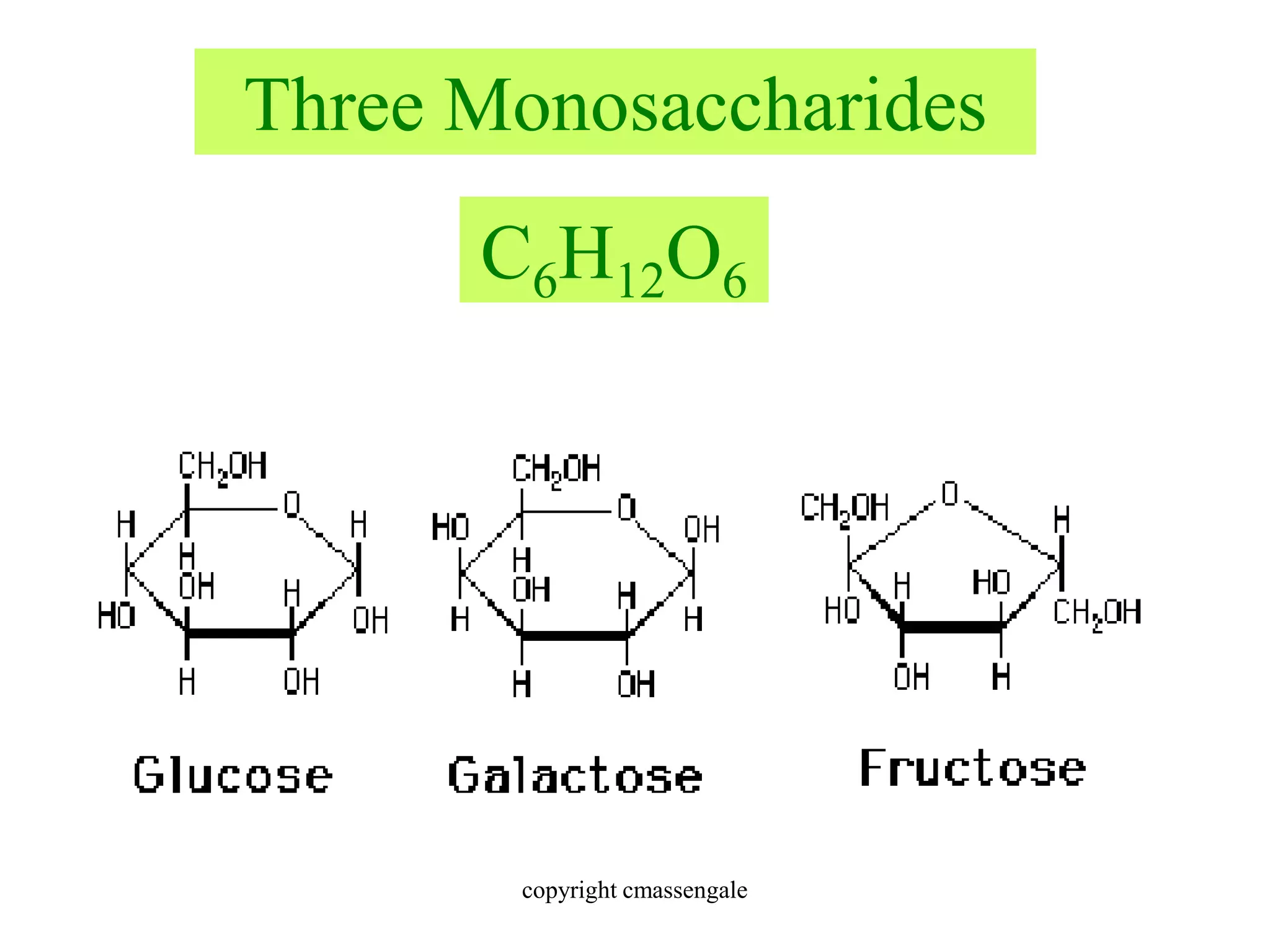
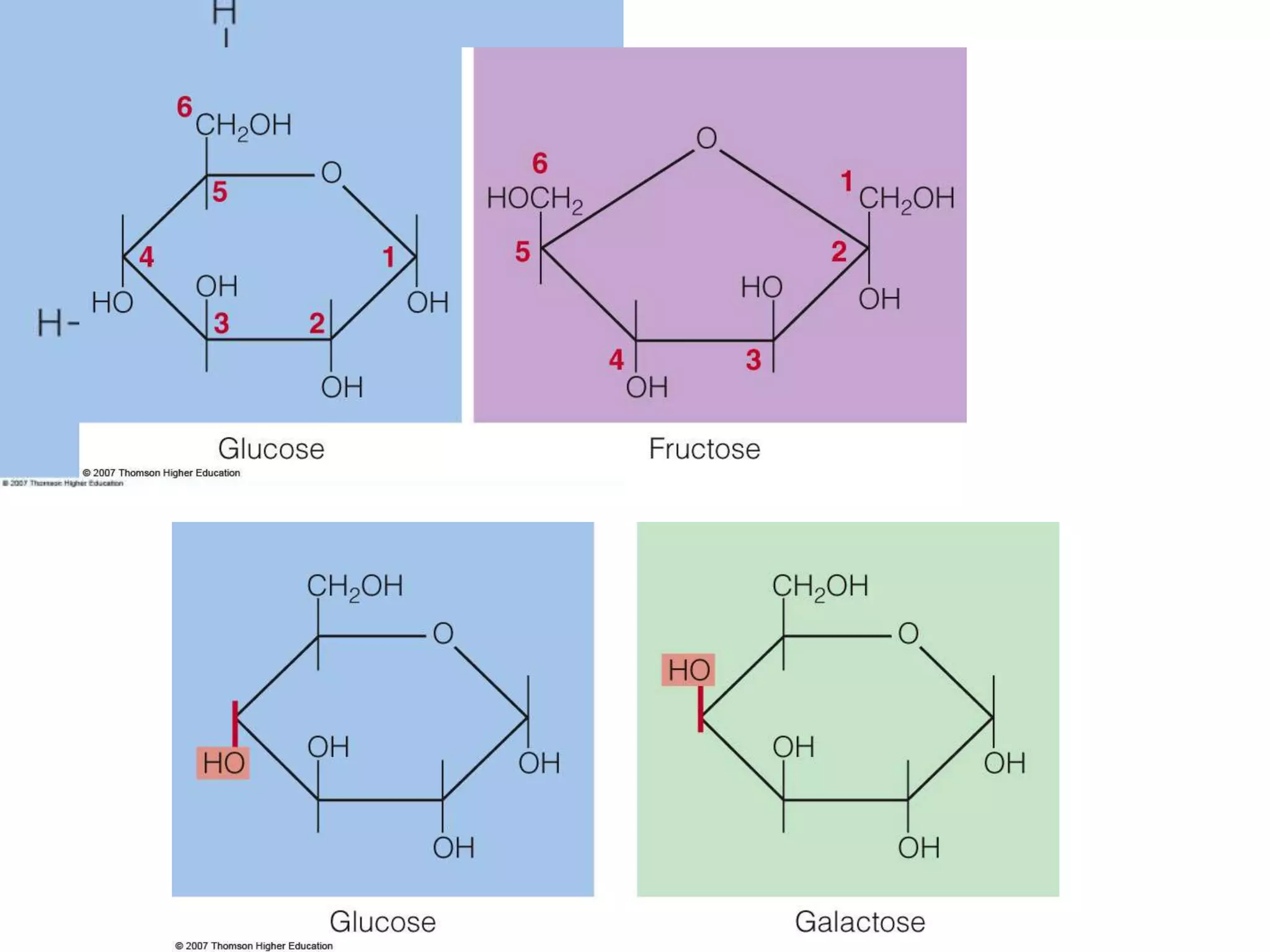
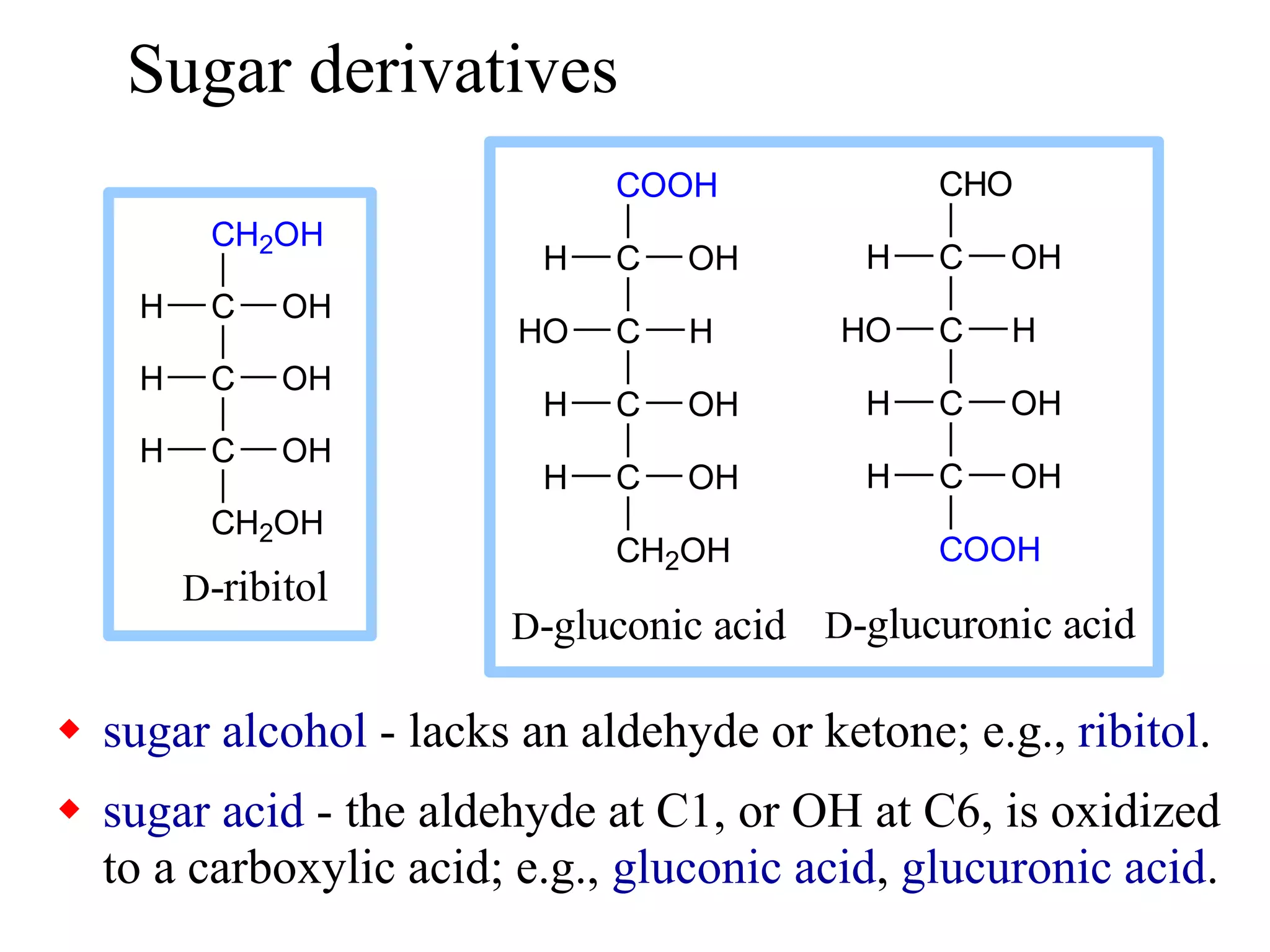
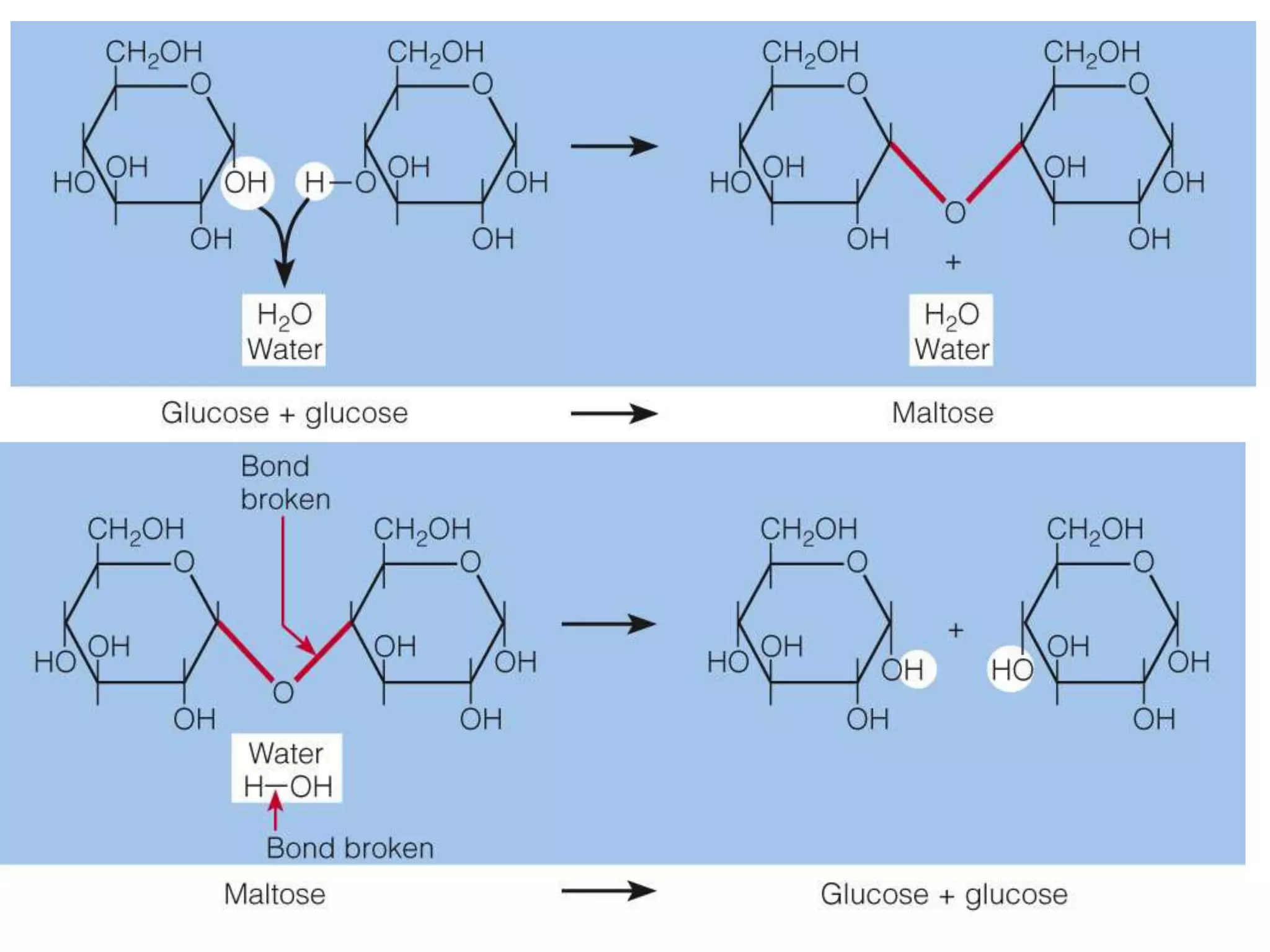
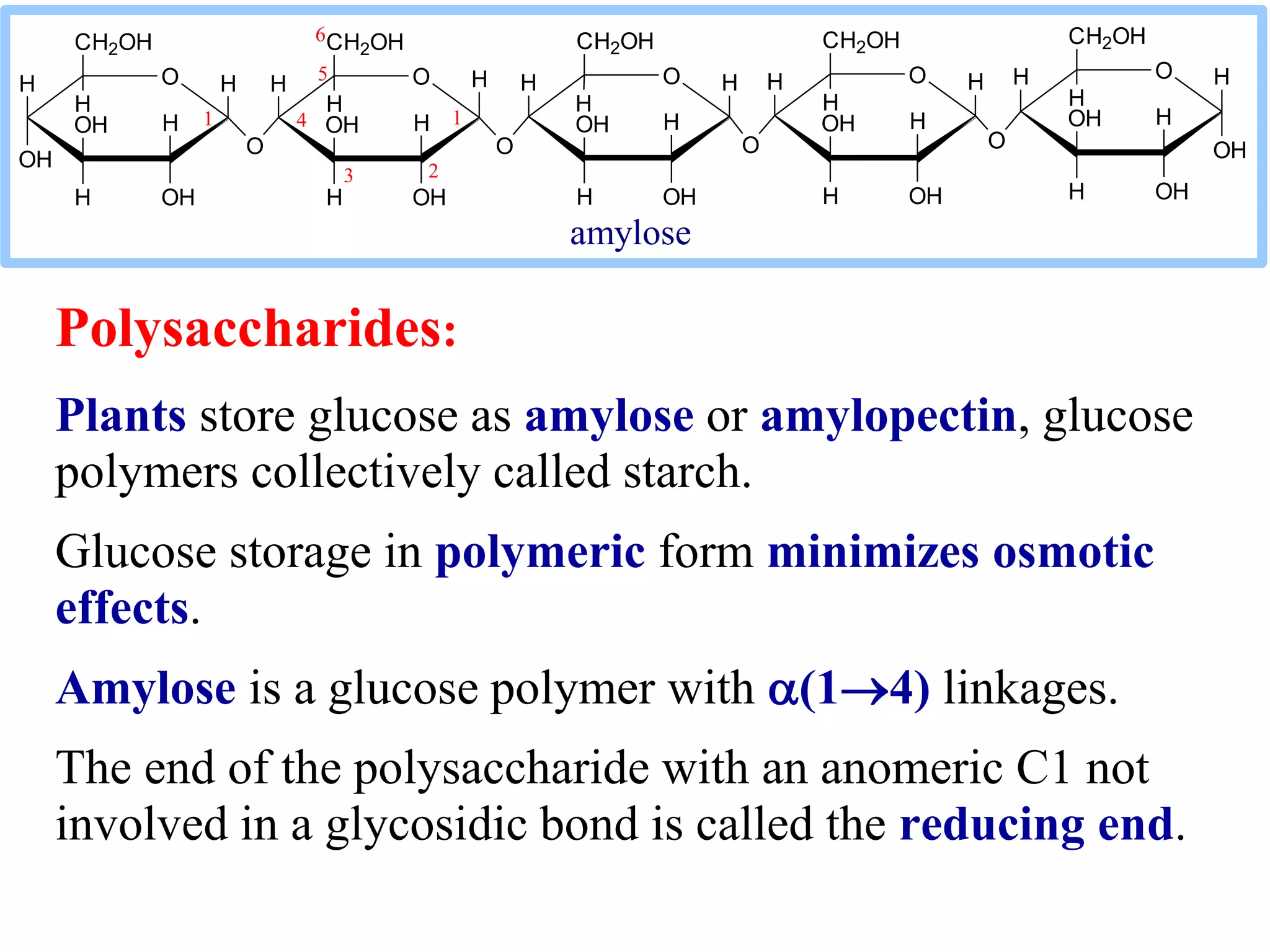
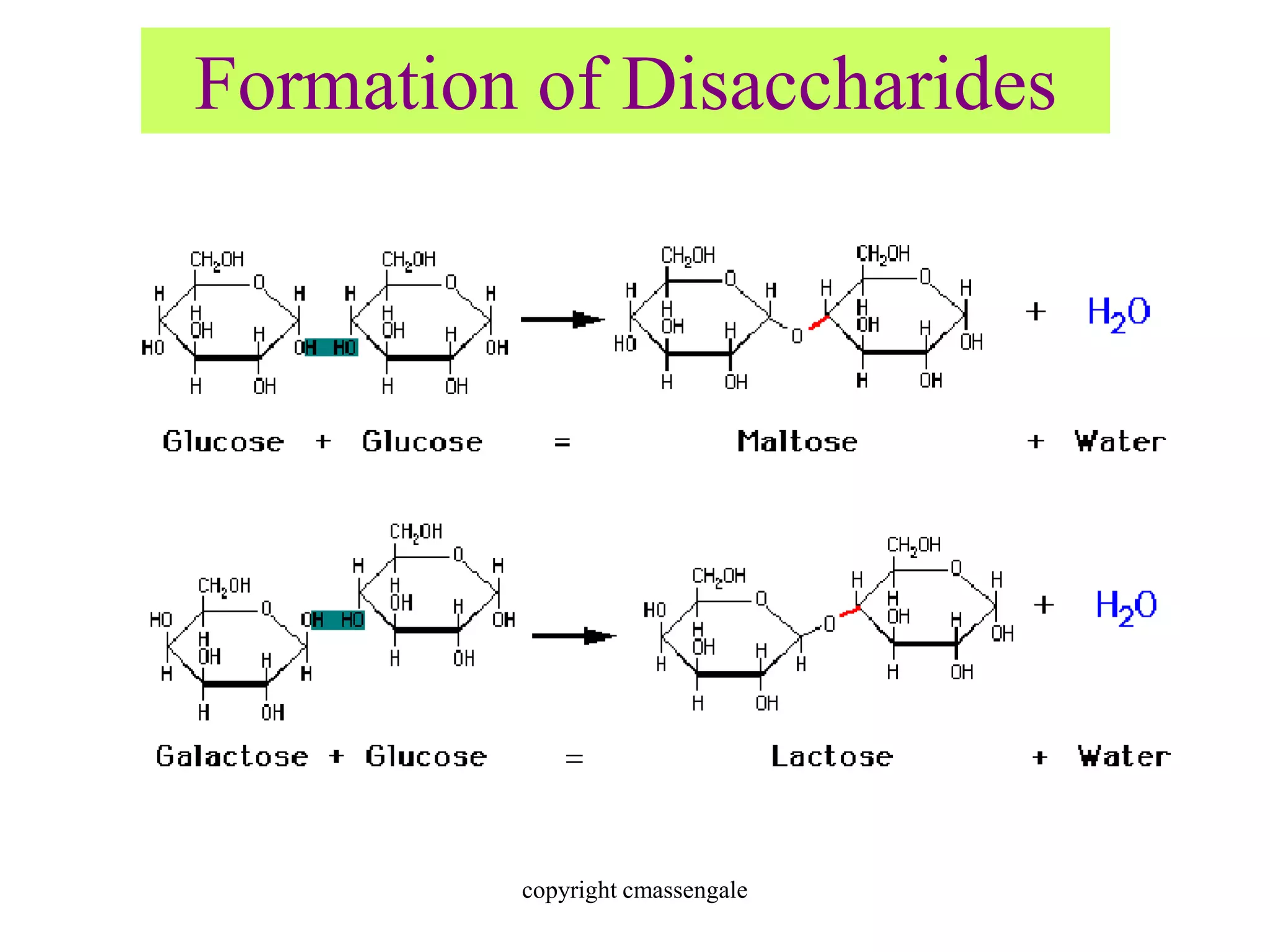
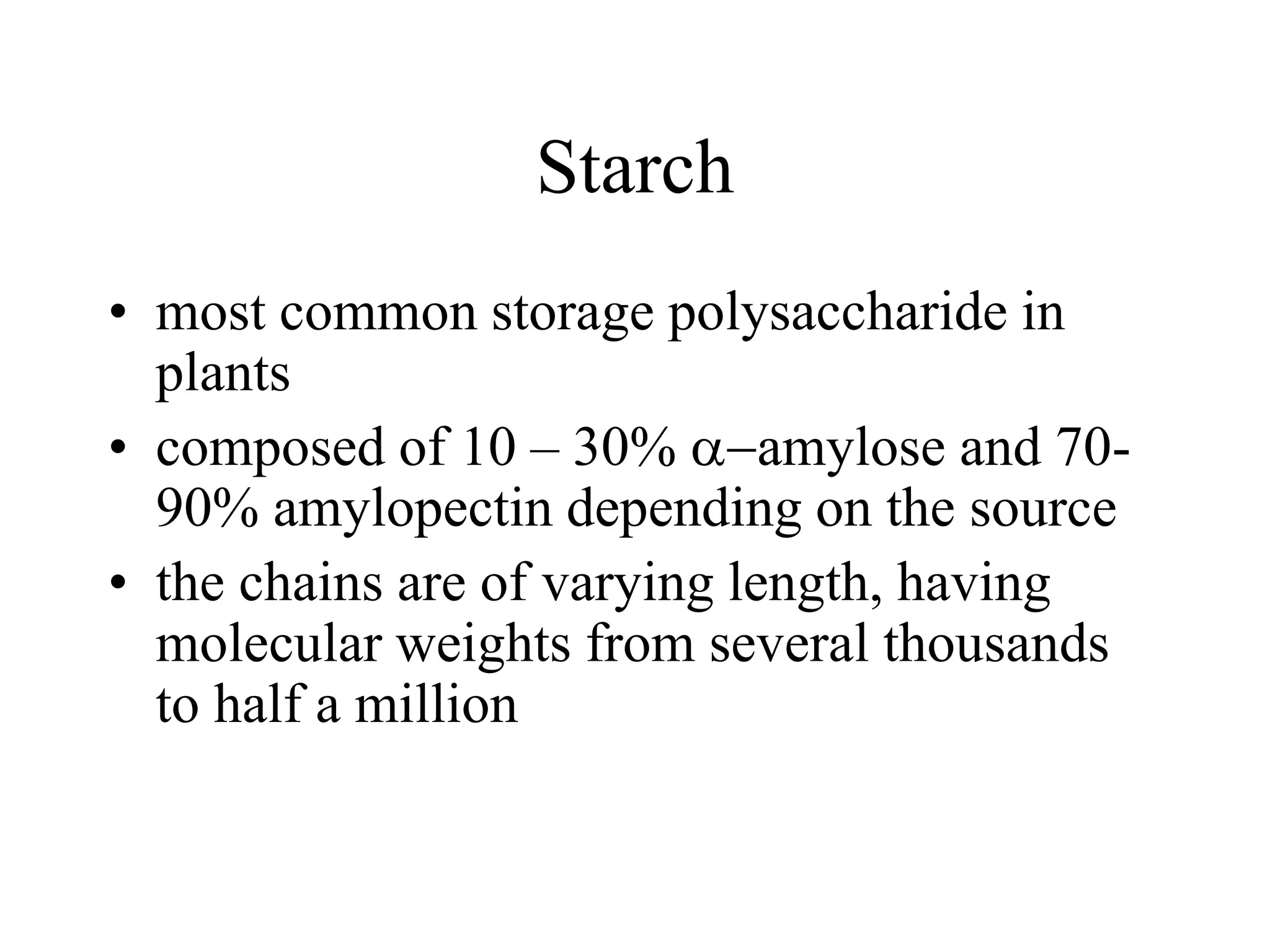
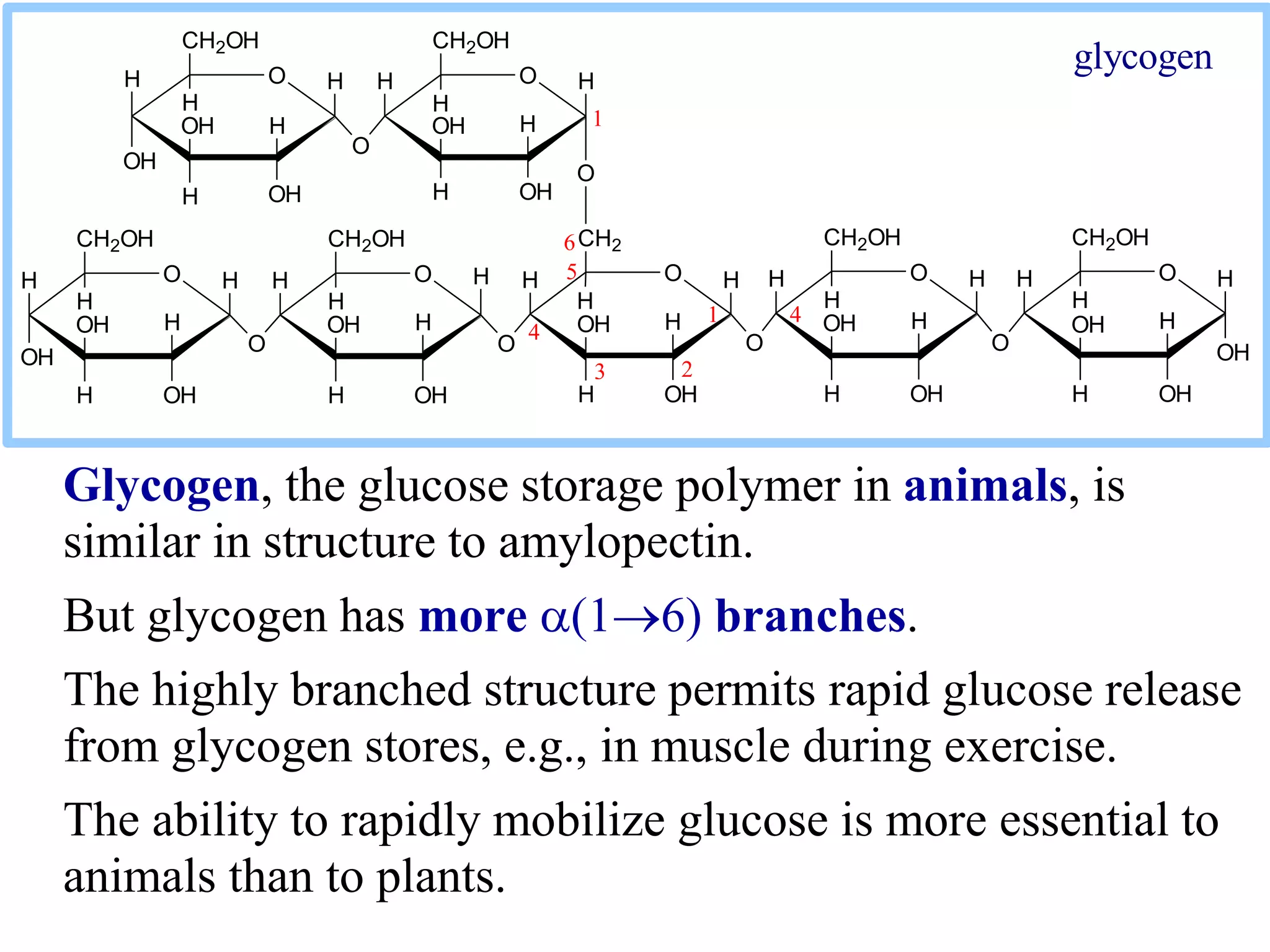
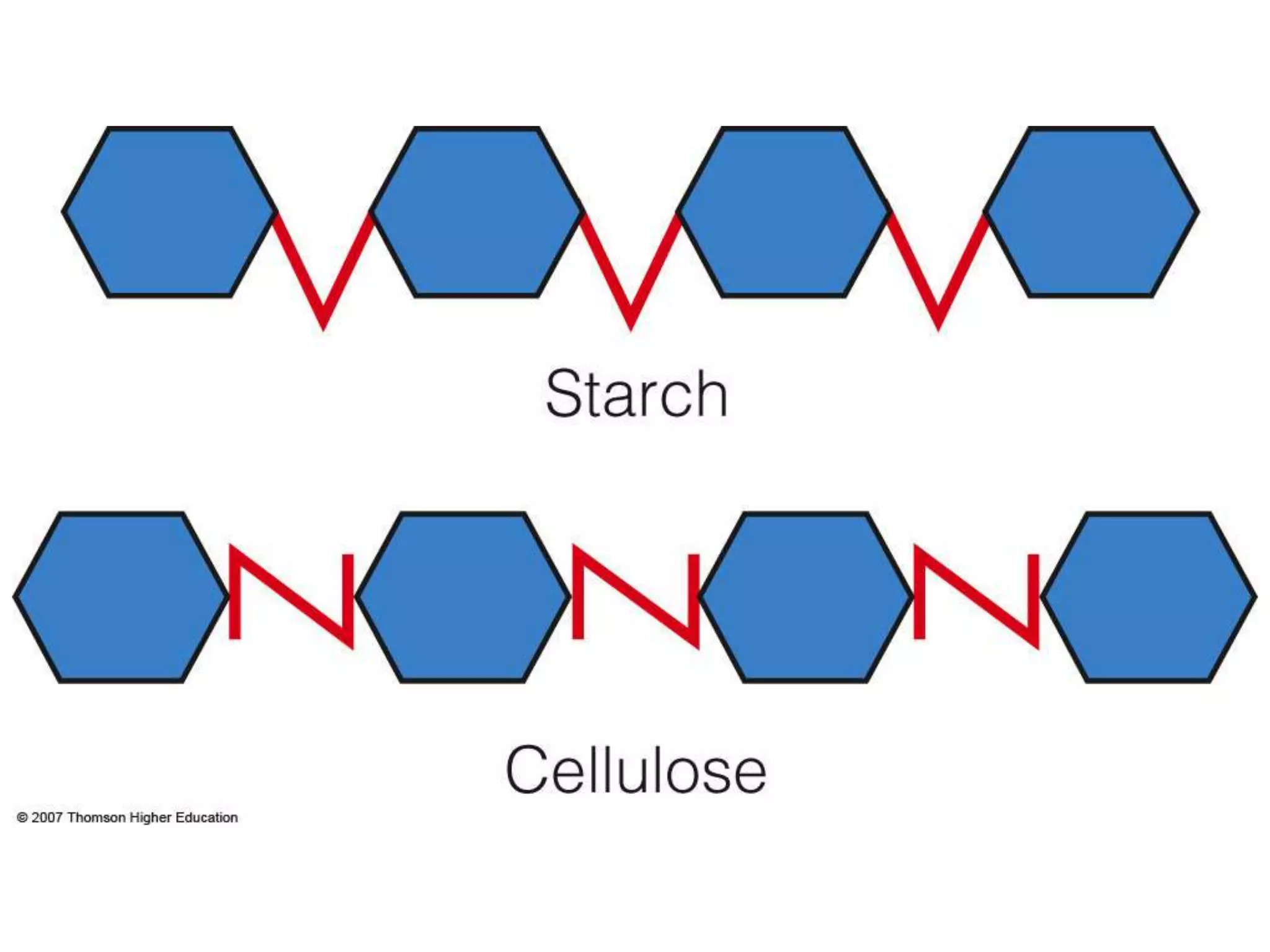
Carbohydrates are polyhydroxy aldehydes or ketones that can be hydrolyzed into monosaccharides. They serve important functions like energy storage, structure, and metabolism. Glucose is a key monosaccharide that provides energy through cellular respiration in plants and animals. Carbohydrates exist as monomers, dimers, and polymers. They can form rings through hemiacetal or hemiketal bonds and exist in different cyclic and linear isomers depending on bonding orientation.