Carbohydrates ppt biochemistry pharmacy for students
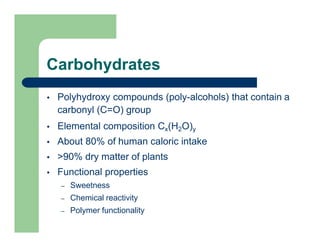
- 1. Carbohydrates Polyhydroxy compounds (poly-alcohols) that contain a carbonyl (C=O) group Elemental composition Cx(H2O)y About 80% of human caloric intake >90% dry matter of plants Functional properties – Sweetness – Chemical reactivity – Polymer functionality
- 3. Monosaccharides Monosaccharides are categorized by the number of carbons (typically 3-8) and whether an aldehyde or ketone Most abundant monosaccharides are hexoses (6 carbons) Most monosaccharides are aldehydes, i.e. aldoses O H C H C OH H H C OH C O aldehyde ketone
- 4. Fisher projections H C OH H H H O H C 4 OH 5 6 C H2OH C1 C2 OH HO C3 H O H C H2OH 1 C 2 HO C 3 H C OH 4 C OH 5 6C H2OH D-fructose (an ketohexose) D-glucose (an aldohexose)
- 7. Cyclic Forms O OH HO OH HO H Lowest energy state H C1 H 2C 4 C 6 CH2OH C5 H C3 H -D-glucopyranose (glucose) —an aldose a hexose an aldohexose —C1 chairconformation -D-fructopyranose (fructose) —a ketose a hexulose a ketohexose —1C chair conformation O C H H OH OH H OH CH2OH 1 2C C3 H C OH 4 5 6C H
- 8. Ring Nomenclature pyranose is a six-membered ring (a very stable form due to optimal bond angles) furanose is a five-membered ring
- 9. Chirality Geometric property of a rigid object (or spatial arrangement of atoms) of being non-super-imposable on its mirror image H H C OH HO C H H C OH its mirror image is an "optical isomer" C OH CH2OH H C O 4 chiral centeres.g. atC2 carbon: This structure has a non- superimposable mirror image CHO (CHOH)3CH2OH H C2 OH
- 10. Isomers Isomers are molecules that have the same chemical formula but different structures Stereoisomer differs in the 3-D orientation of atoms Diastereomers are isomers with > 1 chiral center. – Pairs of isomers that have opposite configurations at one or more of the chiral centers but that are not mirror images of each other. Epimers are a special type of diastereomer. – Stereoisomers with more than one chiral center which differ in chirality at only one chiral center. – A chemical reaction which causes a change in chirality at one one of many chiral center is called an epimerisation.
- 11. Enantiomers Isomerism in which two isomers are mirror images of each other. (D vs L)
- 13. Anomer An anomer is one of a special pair of diastereomeric aldoses or ketoses – differ only in configuration about the carbonyl carbon (C1 for aldoses and C2 for ketoses)
- 14. Carbonyl Group Carbonyl groups subject to nucleophilic attack, since carbonyl carbon is electron deficient: – -OH groups on the same molecule act as nucleophile, add to carbonyl carbon to recreate ring form
- 15. OH H O O H O H O H OH O O 5 5 5 5 1 1 1 1 anomer anomer Carbonyl carbon freely rotates O can attack eitherside
- 16. Specification of Conformation, chirality and anomeric form of sugars Determination of chair conformation – Locate the anomeric carbon atom and determine if numbering sequence is clockwise (n= +ve) or counterclockwise (n= -ve). – Observe if the puckered ring oxygen atom lies “above” (p= +ve) the plane of the ring or below (p= -ve). – Multiply n*p. If the product is +ve then C1, -ve then 1C Determination of chiral family – Locate the reference carbon atom contained within the ring and determine whether the bulky substituent (OH or CH2OH) is equatorial (r= +ve) or axial (r= -ve). – Multiply n*p*r. If product is +ve the chiral family is D, when it is –ve the chiral family is L
- 17. Determination of Anomeric form: – Determine if the hydroxyl substituent on the anomeric carbon atom is equatorial (a= +ve) or axial (a= -ve). – Multi[ly (n*p) by (n*p*r) by a. When the product is positive, the anomer is ; when the product is negative the anomer is Specification of Conformation, chirality and anomeric form of sugars
- 18. Mutarotation The - and - anomers of carbohydrates are typically stable solids. In solution, a single molecule can interchange between – straight and ring form – different ring sizes – α and β anomeric isomers Process is – dynamic equilibrium – due to reversibility of reaction All isomers can potentially exist in solution – energy/stability of different forms vary
- 19. Mutarotation : interconversion of - and - anomers For example, in aqueous solution, glucose exists as a mixture of 36% - and 64% - (>99% of the pyranose forms exist in solution).
- 20. Anomer Interconverision 80 70 60 50 40 30 20 10 0 % of all isomers D-glucose D-fructose D-mannose D-galactose α-pyranose β-pyranose α -furanose β-furanose Generally only a few isomers predominate
- 21. +57.2o +112o +19o pure -D-(+)-glucopyranose1 [D 66% 34% pure -D-(+)-glucopyranose2 TIME(min)
- 23. OH HO OH O O HO OH OH O HO OH OH OH H C OH OH H OH HO OH O OH OH OH OH OH H CH2OH HO OH O H Mutarotation of ribose hydrate (0.09%) H H H OH CH2OH keto-form (0.04%) -pyranose (20.2%) -furanose (7.4%) HO -pyranose (59.1%) -furanose (13.2%)
- 24. Stability of Hemiacetals/Hemiketals As general rule the most stable ring conformation is that in which all or most of the bulky groups are equatorial to the axis of the ring
- 25. Reactions Isomerization glucose Oxidation R-CHO R-CH2OH fructose mannose R-COOH R-COOH Reduction sugar sugar alcohols Acetal formation sugar glycoside Browning reactions O H C H C OH HO C H H C OH H C OH CH2OH carbonyl group is key
- 26. Isomerization Isomerization is possible because of the “acidity” of the hydrogen O H C OH H C OH CH2OH hydrogen H C H C OH (on C next to carbonyl)HO C H O O H C C OH HO C H H C OH H C OH CH2OH keto form base H C C OH HO C H H C OH H C OH CH2OH enol form
- 27. Isomerization O 2 C H OH H C H C OH HO C H H C OH H C OH O 2 C H OH H HO C H H C OH H C OH H C HO C C O CH2OH HO C H H C OH H C OH CH2OH D-fructose D-glucose D-mannose
- 28. Oxidation/Reduction Oxidation Increase oxygen or decrease hydrogen Increase oxidation state Remove electrons Reduction Decrease oxygen or increase hydrogen Decrease oxidation state Add electrons
- 29. Oxidation Carbonyl group can be oxidized to form carboxylic acid Forms “-onic acid” (e.g. gluconic acid) Can not form hemiacetal Very hydrophillic – Ca gluconate Can react to form intramolecular esters: – lactones
- 30. Oxidation Also possible to oxidize alcohols to carboxylic acids – “-uronic acids” Galacturonic acids Pectin Reactivity – Aldehydes are more reactive than ketones In presence of base ketones will isomerize Allows ketones to oxidize
- 31. Reducing sugars Reducing sugars are carbohydrates that can reduce oxidizing agents Sugars which form open chain structures with free carbonyl group Reduction of metal ions – Fehling test: CuSO4 in alkaline solution
- 32. Reduction Carbonyl group can be reduced to form alcohol – hydrogenation reaction Forms sugar alcohol (“-itol”) – glucose – mannose – xylose glucitol (aka sorbitol) mannitol xylitol Sweet, same calories as sugar, non-cariogenic Very hydrophillic Good humectants
- 34. Stability of acetals Pyranose >>>> Furanose β -glycosidic > α-glycosidic 1,6>1,4>1,3>1,2 Allow to predict stability of glycosidic linkages in terms of their resistance to hydrolysis – Gentiobiose
- 35. Acid catalyzed Rxns Acid hydrolysis of hemiactals and hemiketals (mutarotation) Anhydro sugars – 1C conformation Reversion sugars – Formation of oligosaccharides under conditions of high sugar concentration, dilute acid……. Maple syrup, fruit juice concentrates – Detection of invert sugar in juices/honey Enolization and Dehydration – Formation of 3-deoxyosones and HMF/furfural
- 36. Hydrolysis of hemiactals and hemiketals (mutarotation) – Base catalyzed loss of H from anomeric –OH Acetals and Ketals are stable – Sugar esters will be hydrolyzed in alkali Enolization – Favored by alkali Reduction of metal ions – Alkali prevents hydrolysis of non-reducing sugar Base catalyzed Rxns
- 39. What is Biochemistry? • Biochemistry = chemistry of life. • Biochemists use physical and chemical principles to explain biology at the molecular level. • Basic principles of biochemistry are common to all living organism
- 40. How does biochemistry impact you? • Medicine • Agriculture • Industrial applications • Environmental applications
- 41. Principle Areas of Biochemistry • Structure and function of biological macromolecules • Metabolism – anabolic and catabolic processes. • Molecular Genetics – How life is replicated. Regulation of protein synthesis
- 42. Once upon a time, a long long time ago….. Vitalism: idea that substances and processes associated with living organisms did not behave according to the known laws of physics and chemistry Evidence: 1) Only living things have a high degree of complexity 2) Only living things extract, transform and utilize energy from their environment 3) Only living things are capable of self assembly and self replication
- 43. Origins of Biochemistry: A challenge to “Vitalism.” Famous Dead Biochemist!
- 44. Fallacy #1: Biochemicals can only be produced by living organisms •Dead Biochemist #1 •1828 Friedrich Wohler
- 45. Fallacy #2: Complex bioconversion of chemical substances require living matter Dead Biochemists #2 •1897 Eduard Buchner Glucose + Dead Yeast = Alcohol
- 46. Dead Biochemists #3 • Emil Fischer Fallacy #2: Complex bioconversion of chemical substances require living matter
- 47. Fallacy #2: Complex bioconversion of chemical substances require living matter Dead Biochemists #4 1926 J.B. Sumner
- 48. Findings of other famous dead biochemist • 1944 Avery, MacLeod and McCarty identified DNA as information molecules • 1953 Watson (still alive) and Crick proposed the structure of DNA • 1958 Crick proposed the central dogma of biology
- 49. Organization of Life • elements • simple organic compounds (monomers) • macromolecules (polymers) • supramolecular structures • organelles • cells • tissues • organisms
- 50. Range of the sizes of objects studies by Biochemist and Biologist 1 angstrom = 0.1 nm
- 51. Most abundant, essential for all organisms: C, N, O, P, S, H Less abundant, essential for all organisms : Na, Mg, K, Ca, Cl Trace levels, essential for all organism: Mn, Fe, Co, Cu, Zn Trace levels, essential for some organisms: V, Cr, Mo, B, Al, Ga, Sn, Si, As, Se, I, Elements of Life
- 52. Important compounds, functional groups
- 53. Many Important Biomolecules are Polymers protein complex protein subunit amino acid membrane phospholipid fatty acid cellw all cellulose glucose c hromos ome DNA monomer polymer supramolecular structure lipids proteins carbo nucleic acids nuc leotide
- 58. Common theme: Monomers form polymers through condensations Polymers are broken down through hydrolysis.
- 60. Prokaryote Cell
- 61. Cellular Organization of an E. coli Cell 200 – 300 mg protein / mL cytoplasm
- 62. Eukaryote Cell