1) Carbohydrates are classified into four main groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Monosaccharides are the simplest form of carbohydrates and include sugars like glucose, fructose, and galactose.
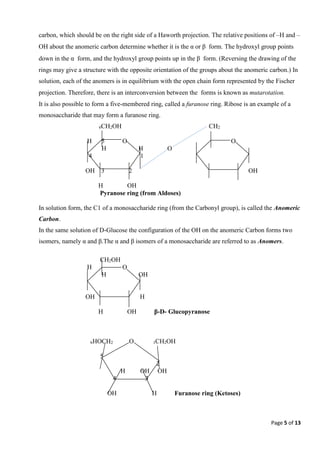
2) Monosaccharides can form ring structures called pyranoses or furanoses via cyclization reactions. This results in two anomeric forms, alpha and beta. Monosaccharides also undergo mutarotation, the interconversion between ring and open-chain forms.
3) Carbohydrates can be further classified based on stereochemistry. Enantiomers are mirror images that rotate polarized light in opposite directions. Diastereomers







![Page 9 of 13
are used in two reaction steps, thus oxidizing the first two C atoms. The osazones are more easily
handled than the sugars themselves, since sugars tend to form syrups when not completely pure.
Both reducing and non-reducing monosaccharides form osazones.
B. Disaccharides
They are formed when two monosaccharide units, same or different react through condensation
polymerisation. They are crystalline solids, soluble in water and sweet in taste.
The monosaccharide units in the disaccharide molecule are linked by Glycosidic bond.
There are two types of disaccharides, namely;
i) Non-reducing sugars
ii) Reducing sugars
a) Non-reducing sugars
They are formed when a glycosidic bond is established between two anomeric (carbonyl) Carbon
atoms, the disaccharide formed is non-reducing e.g. sucrose and trehalose.
(Please draw the structures of the following compounds)
i. Trehalose [O-α-D-Glucopyranosyl-(1, 1)-α-D-Glucopyranose]
ii. Sucrose [O-β-D-Fructofuranosyl-(2, 1)-α-D-Glucopyranose]
b) Reducing sugars
Glucose H2NNHC6H5
CH2NNHC6H5
OH
H
H
HO
OH
H
OH
H
CH2OH
H2NNHC6H5
-C6H5NH2
-NH3
CH=NNHC6H5
O
H
HO
OH
H
OH
H
CH2OH
H2NNHC6H5
-H2O
CH2NNHC6H5
NNHC6H5
H
H
HO
OH
H
OH
H
CH2OH
Glucosazone
Carbonyl function](https://image.slidesharecdn.com/chapter1-carbohydartes-220526065333-a72686e3/85/CHAPTER-1-CARBOHYDARTES-pdf-9-320.jpg)
![Page 10 of 13
They are formed when a glycosidic bond is established between anomeric and non-anomeric Carbon
atoms of the reacting monosaccharides; e.g. maltose, isomaltose, cellobiose and lactose.
(Please draw the structures of the following compounds)
i. Maltose [O-α-D-Glucopyranosyl-(1, 4)-β-D-Glucopyranose]
ii. Isomaltose [O-α-D-Glucopyranosyl-(1, 6)-α-D-Glucopyranose]
iii. Lactose [O-β-D-Galactopyranosyl-(1, 4)-β-D-Glucopyranose]
iv. Cellobiose [O-β-D-Glucopyranosyl-(1, 4)-α-D-Glucopyranose]
Nomenclature
The systematic names of these disaccharides are based on the monosaccharide units present, the
position of the glycosidic bond and the anomers of the monosaccharides. The general rules applied in
naming organic compounds apply.
Properties of disaccharides
a. Hydrolysis
In the lab, they hydrolyzed by hot dil. Mineral acids to constituent monosaccharides. In the living
cell, this reaction is carried out by enzymes generally known as hydrolases.
b. Oxidation
Disaccharide such as sucrose oxidized by hot conc nitric (V) acid to oxalic acid and water.
Hot Conc HNO3
C12H22O11 6{(HOOCCOOH)} + 5H2O
Sucrose Oxalic acid water
c. Dehydration
In the presence of hot conc sulphuric (VI) acid, sucrose loses water to form black carbon. This is a
dehydration reaction. This is the Molisch test for Carbohydrates.
Hot Conc H2SO4
C12H22O11 12C + 11H2O
Sucrose Carbon water
d. Decomposition reaction
With hot conc hydrochloric acid, sucrose decomposes to laevulinic acid.
hot ConcHCl
C12H22O11 CH3-CO-CH2-CH2-COOH
Sucrose Laevulinic acid
e. Fermentation
Sucrose ferments in the presence of yeast to yield alcohol ethanol
yeast](https://image.slidesharecdn.com/chapter1-carbohydartes-220526065333-a72686e3/85/CHAPTER-1-CARBOHYDARTES-pdf-10-320.jpg)


