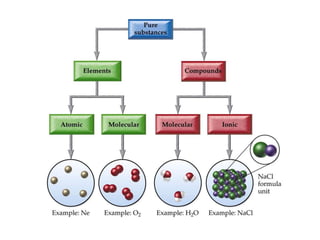
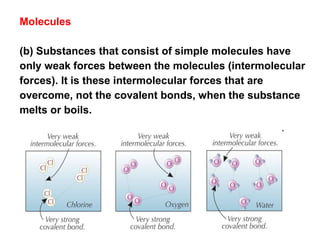
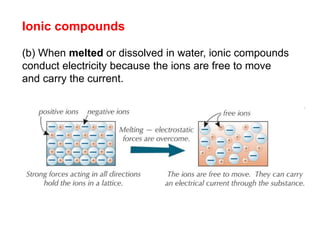
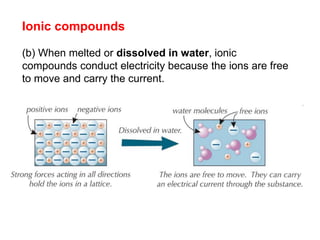
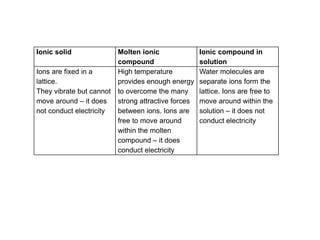
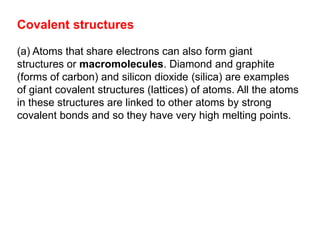
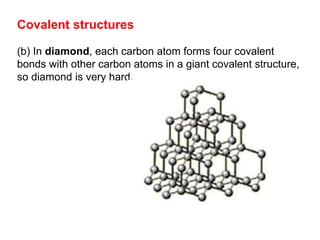
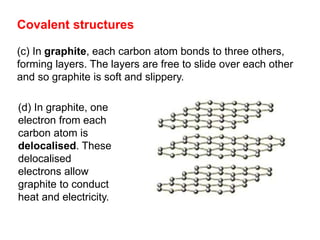
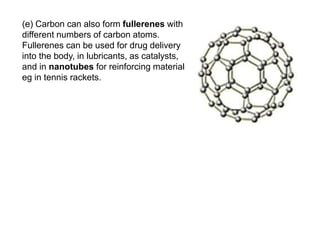
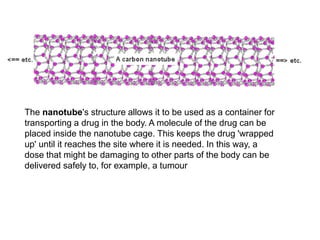
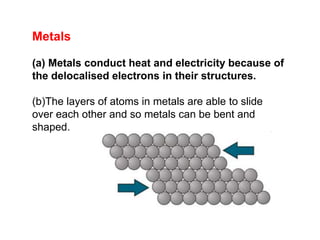
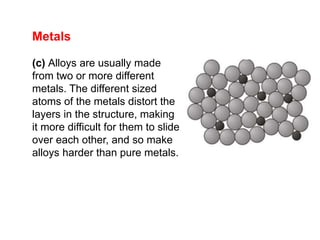
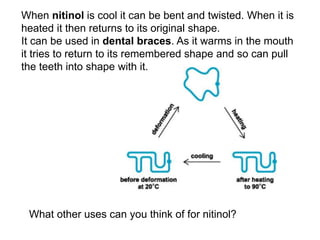
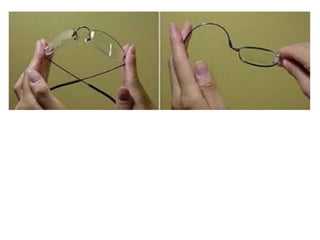
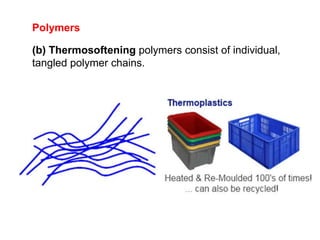
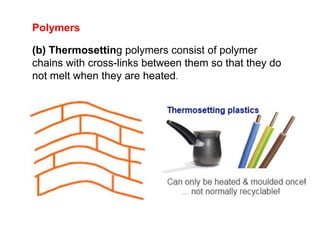
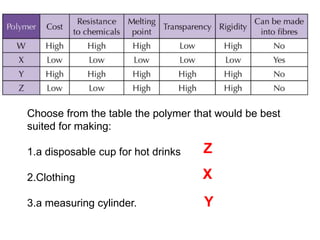
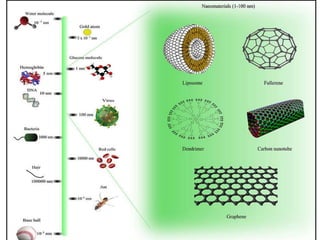
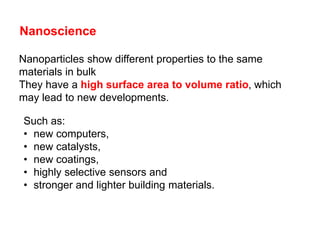
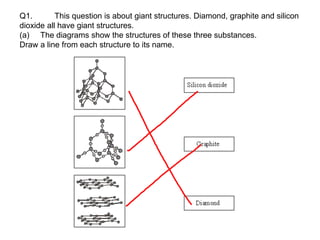
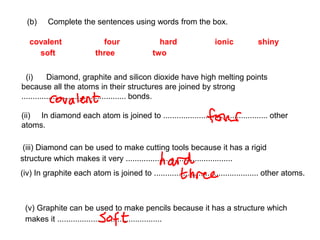
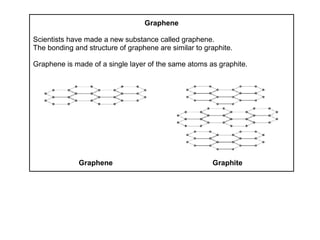
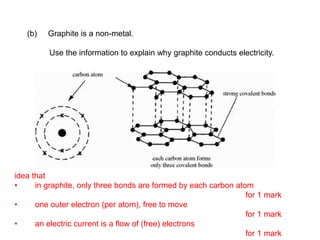
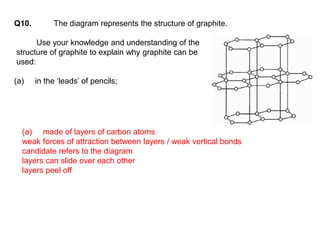
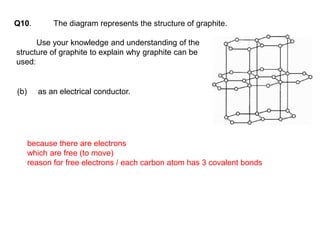
Substances with simple molecular, giant ionic and giant covalent structures have very different properties. Ionic, covalent and metallic bonds are strong, while forces between molecules are weaker. Nanomaterials have new properties due to their small size on the scale of 10 atoms. The structures of substances influence their properties and uses.