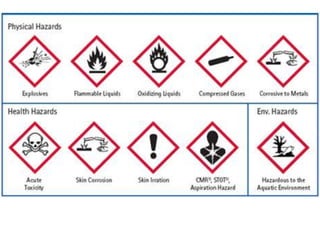
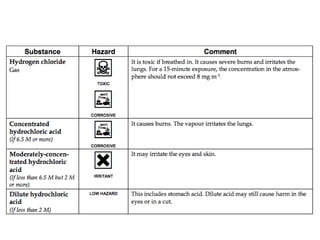
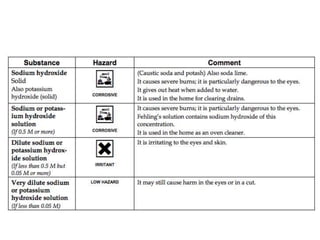
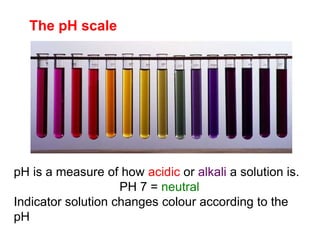
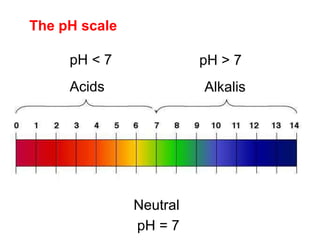
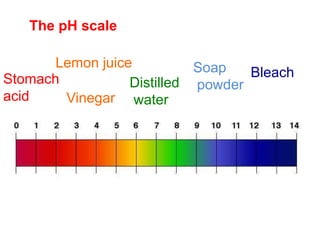
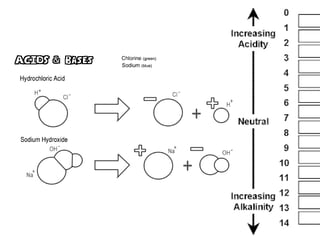
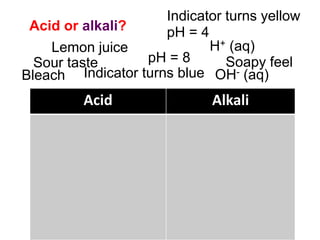
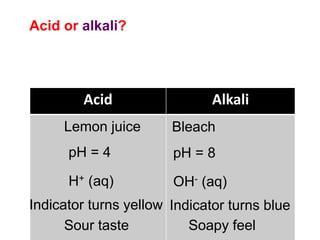
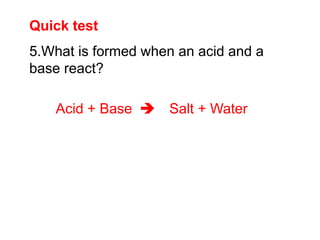
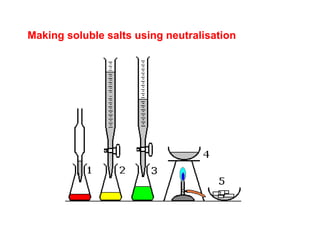
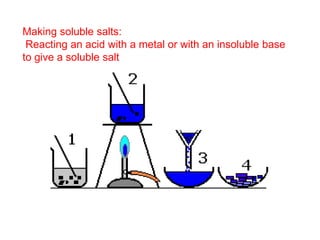
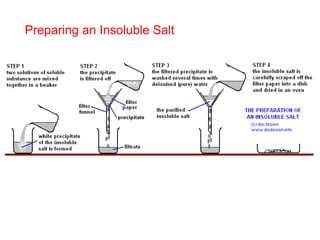
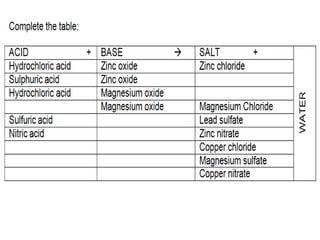
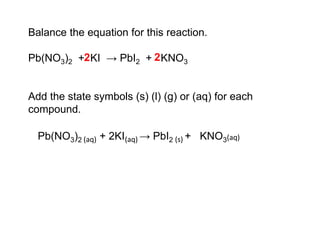
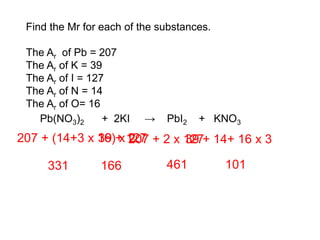
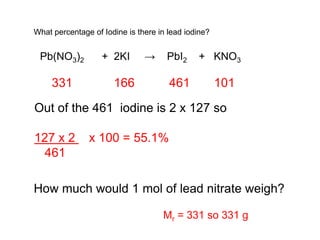
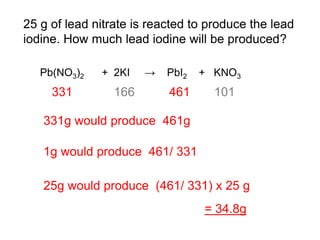
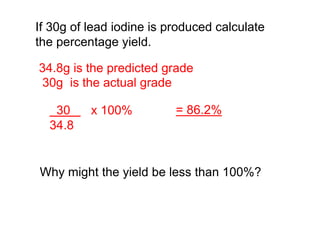
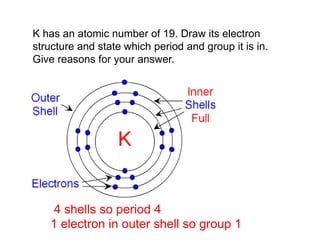
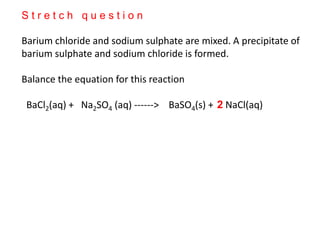
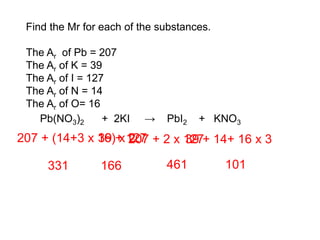
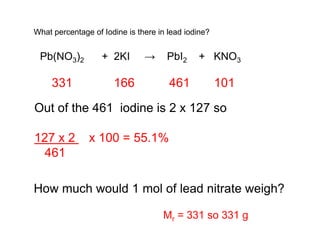
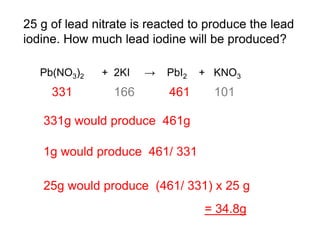
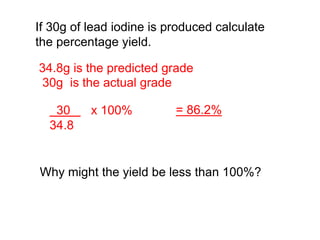
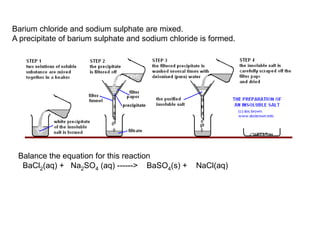
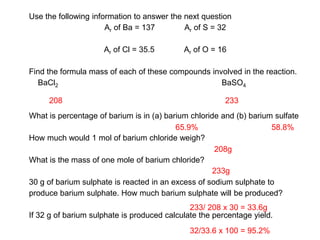
This document discusses acids, bases, and salts. It defines pH and the pH scale, explaining that acids have a pH below 7 while bases have a pH above 7. Neutral solutions have a pH of 7. Acids contain H+ ions while bases contain OH- ions. When an acid and base react, they form a salt and water through neutralization. Common acids and bases are given along with examples of acid-base reactions and properties. Methods for making soluble salts by reacting acids with metals or insoluble bases are also described.