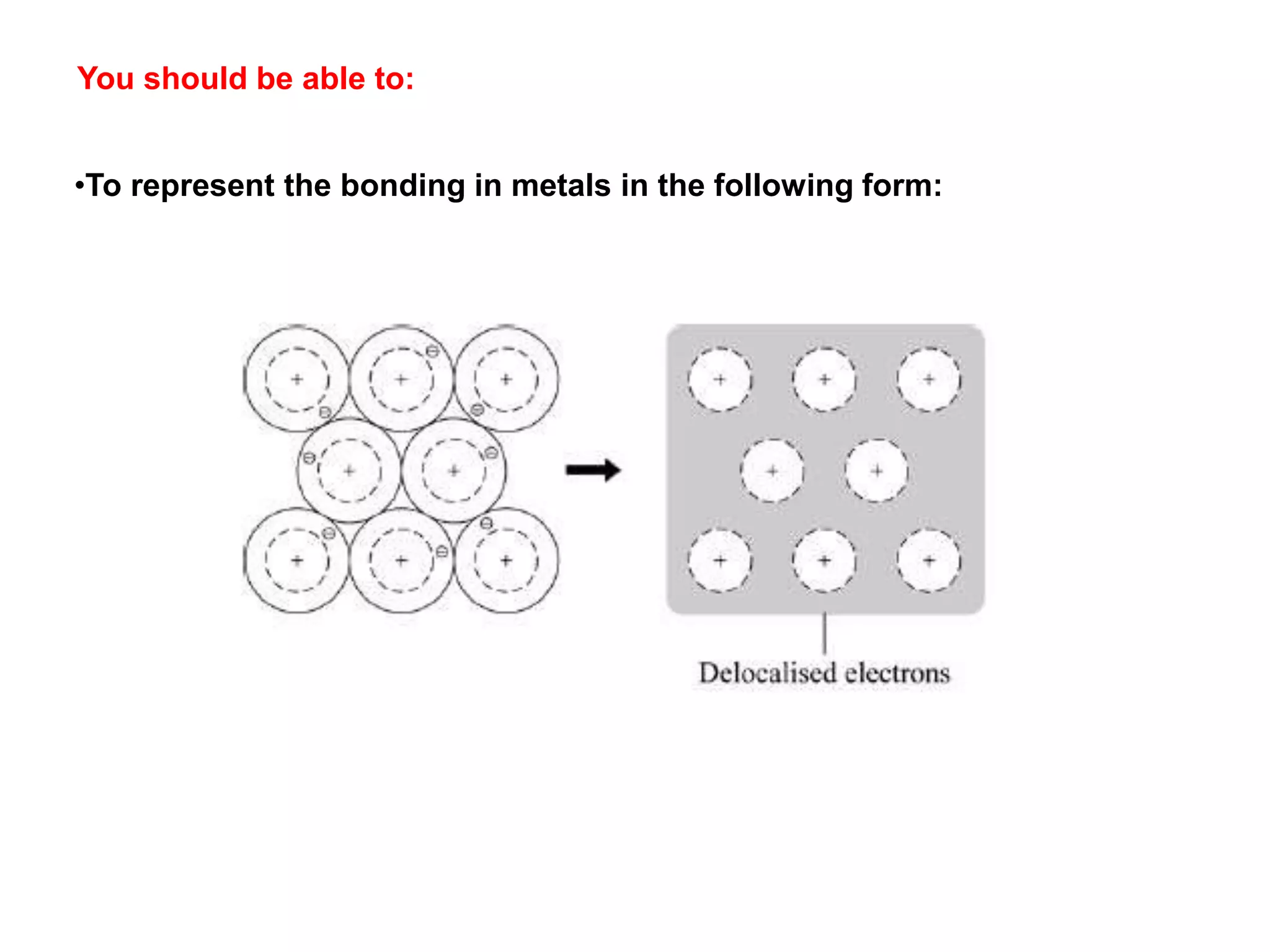
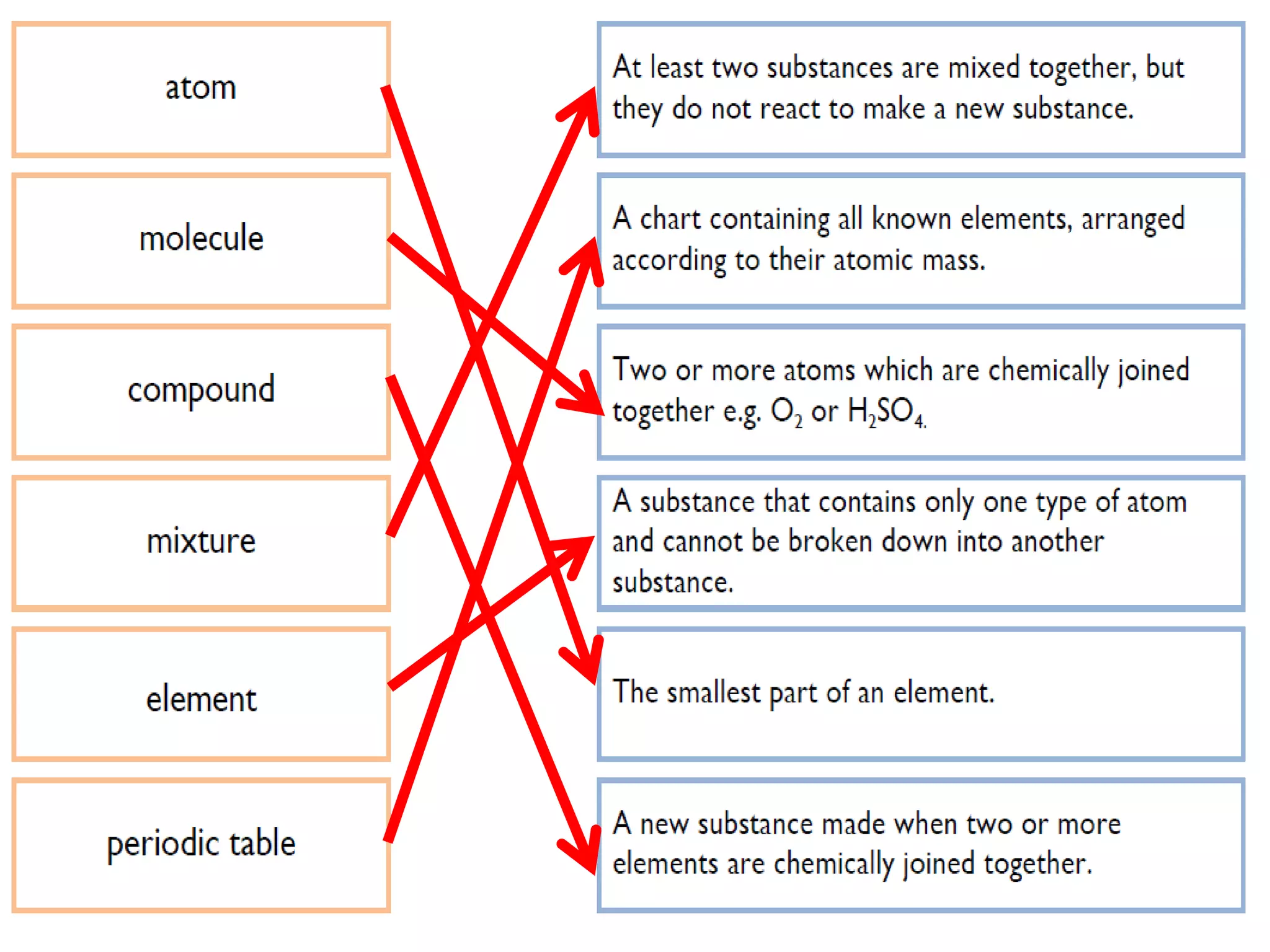
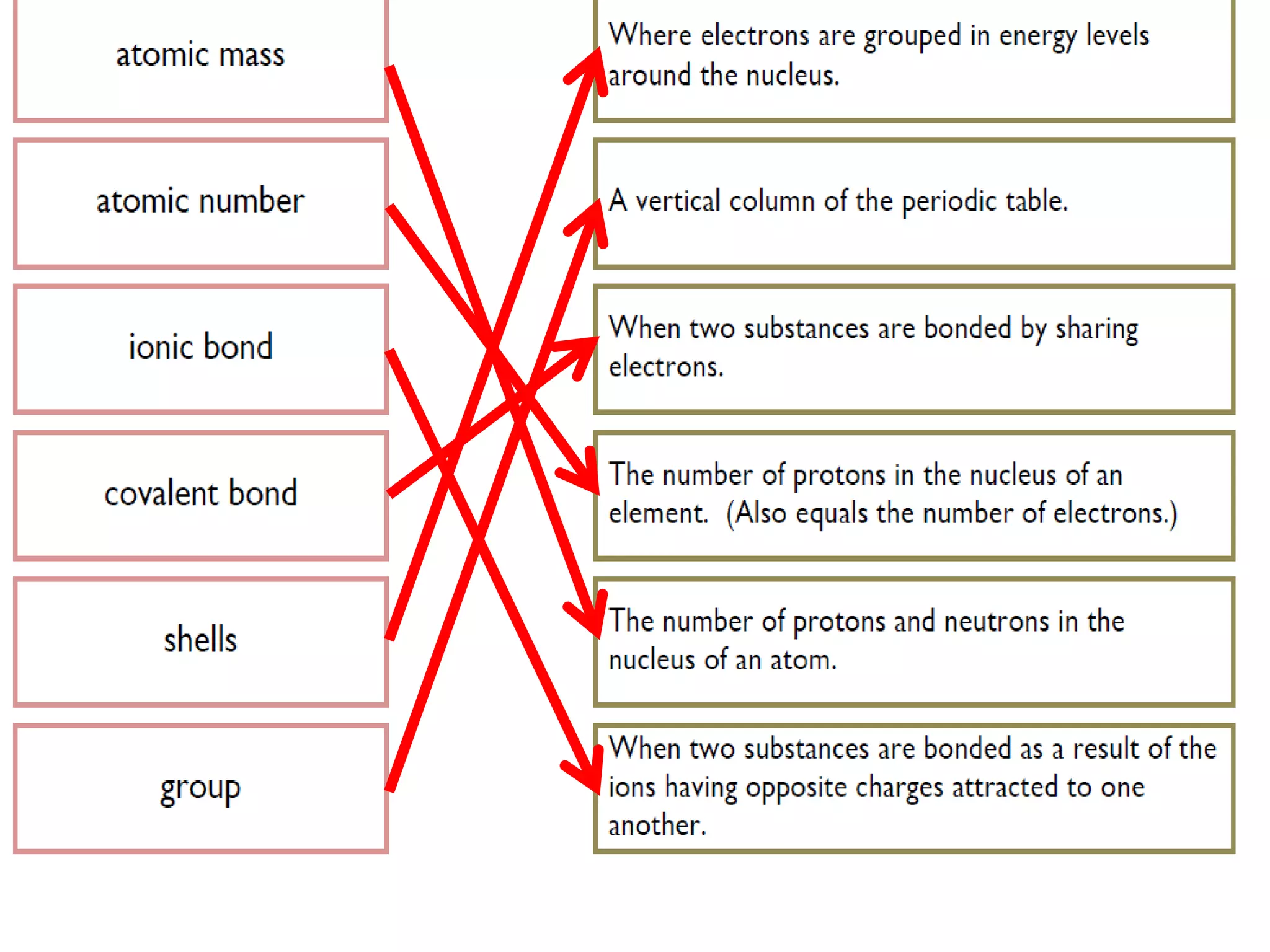
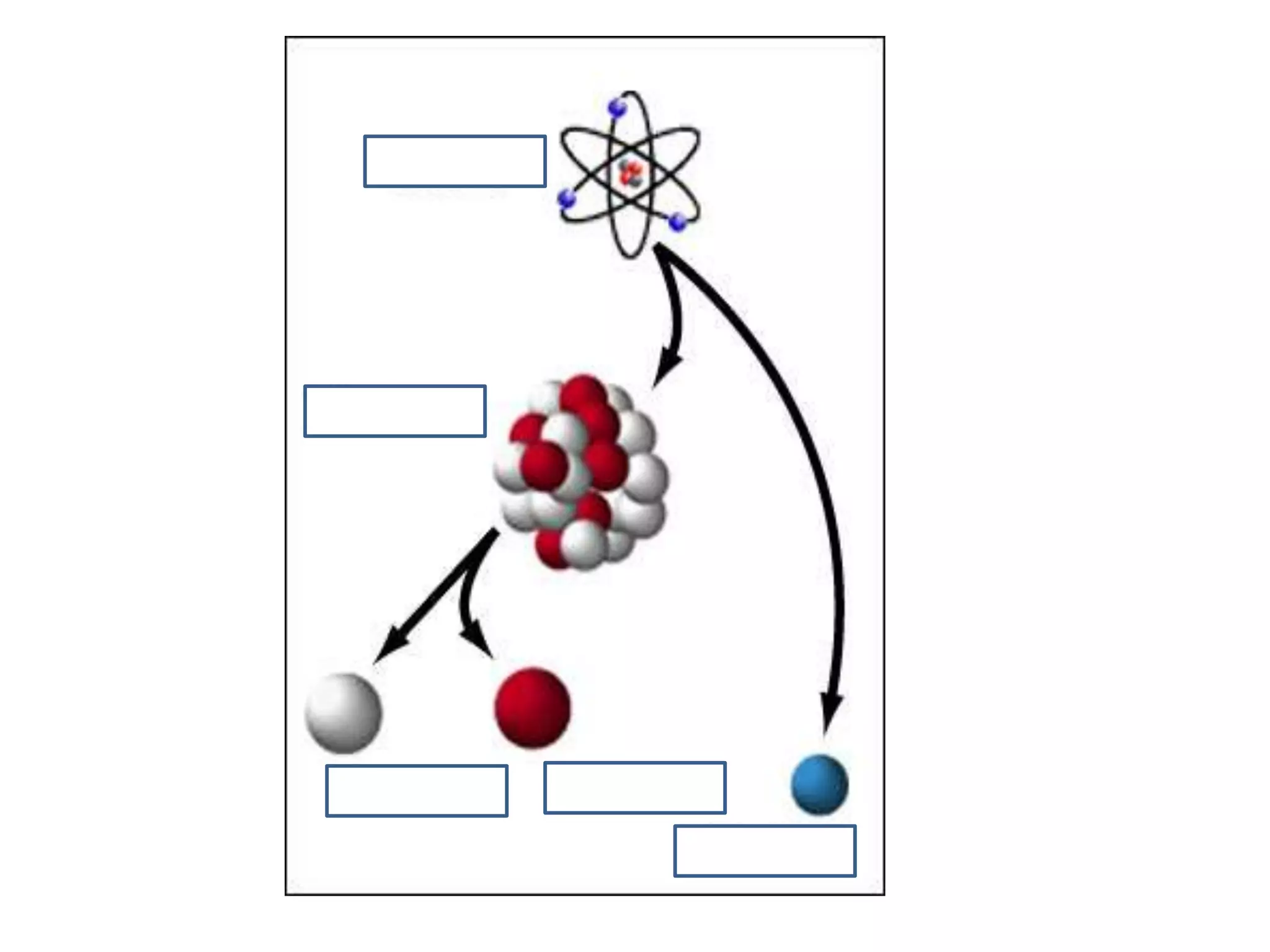
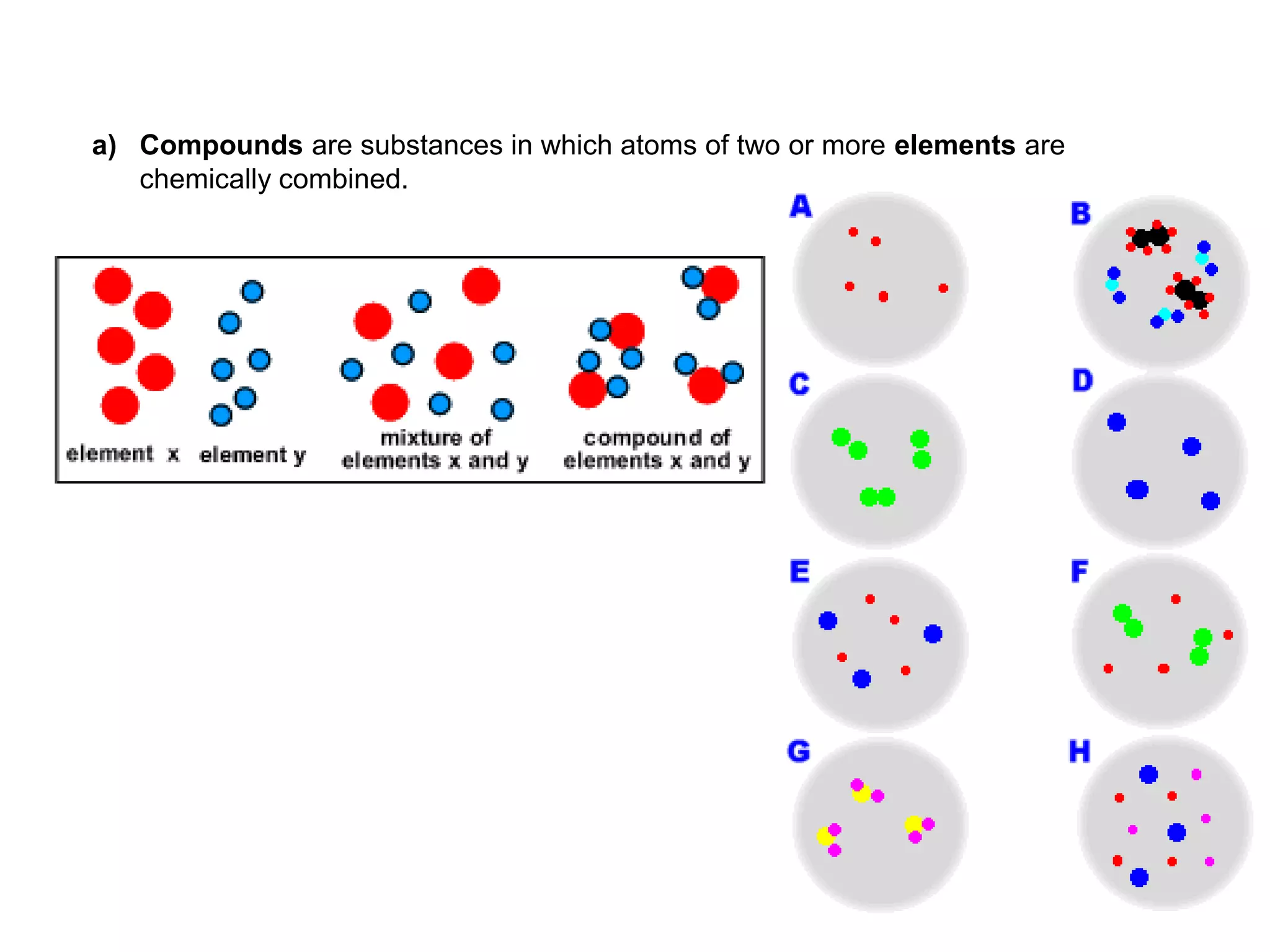
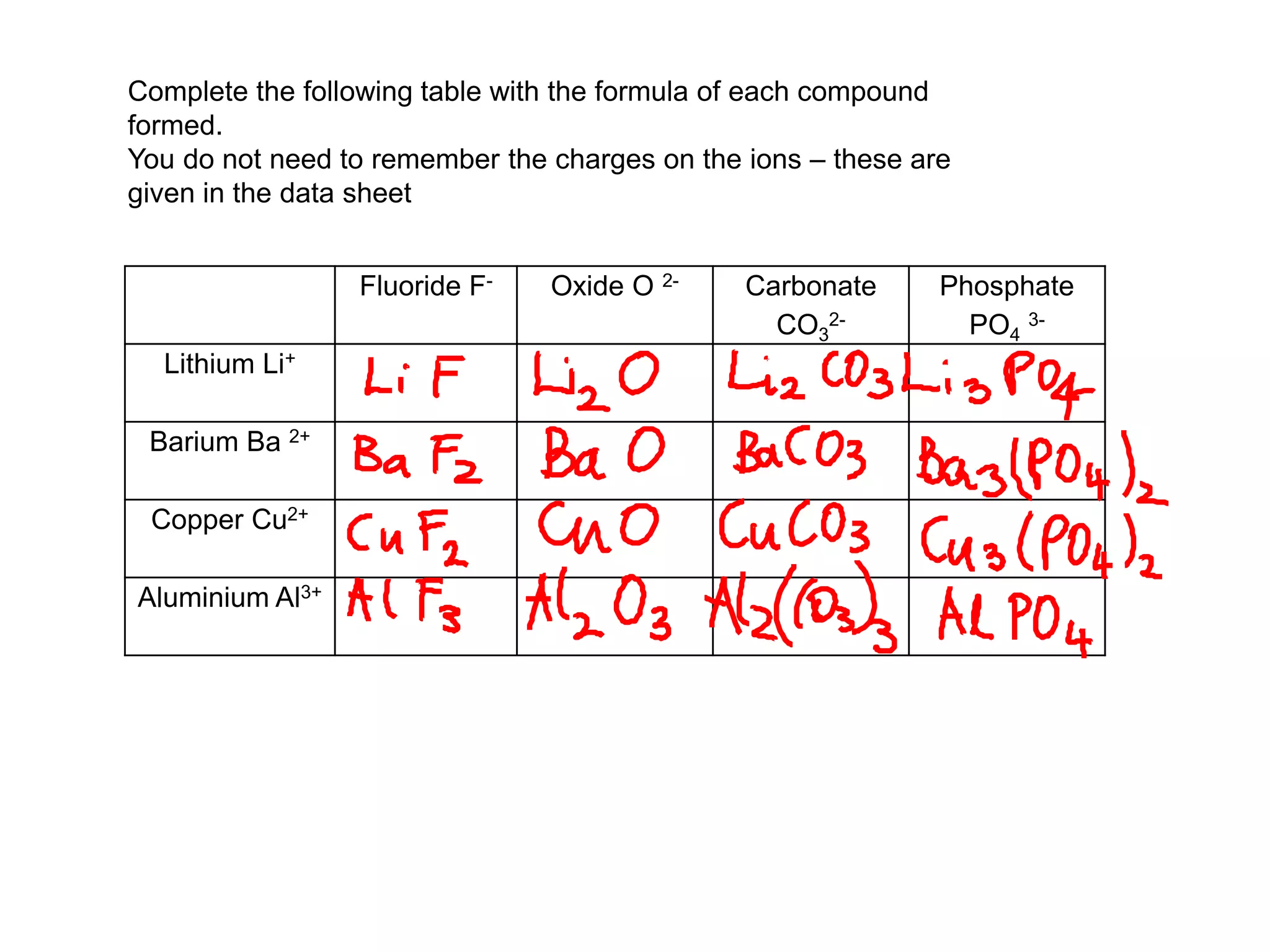
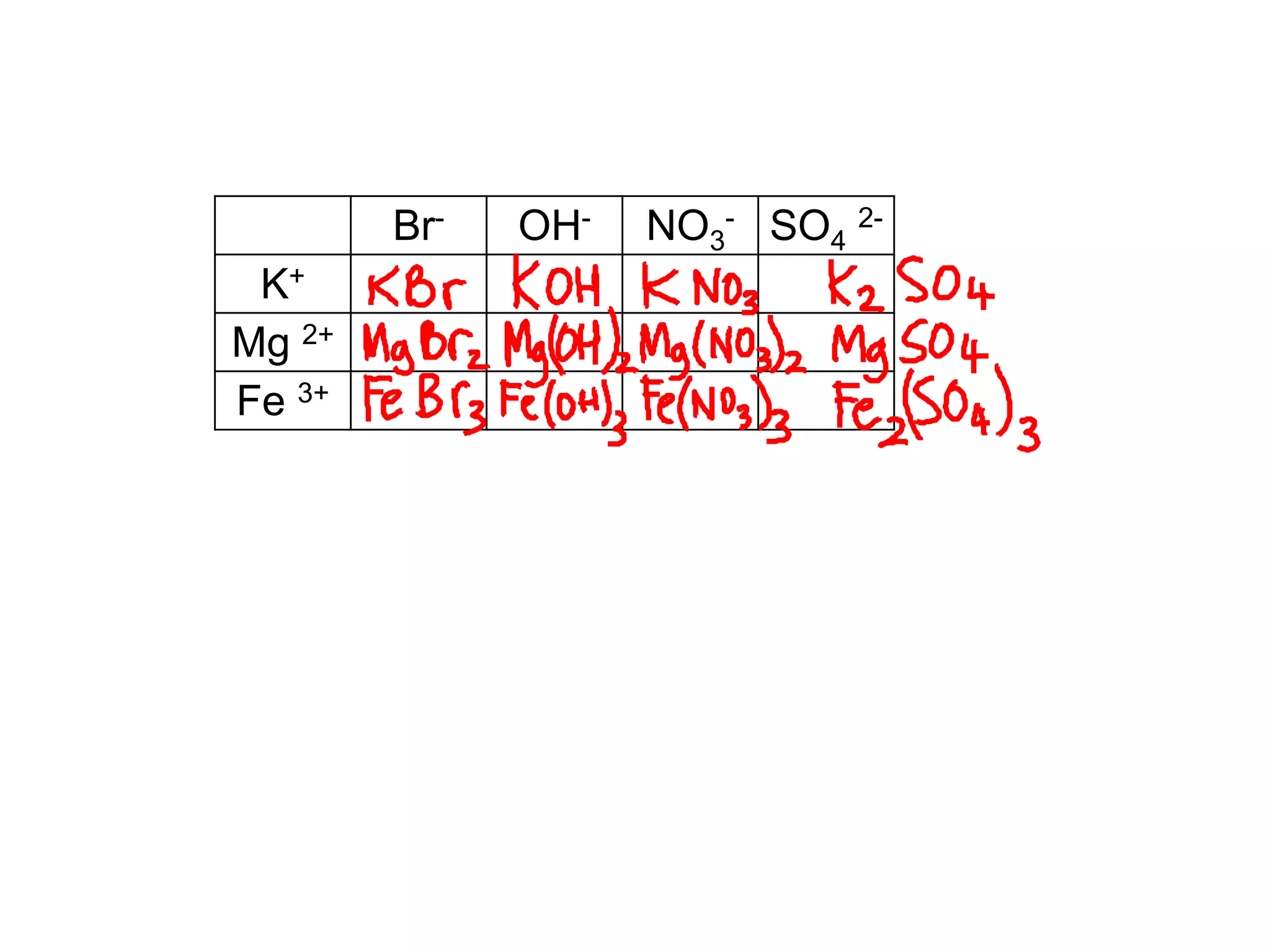
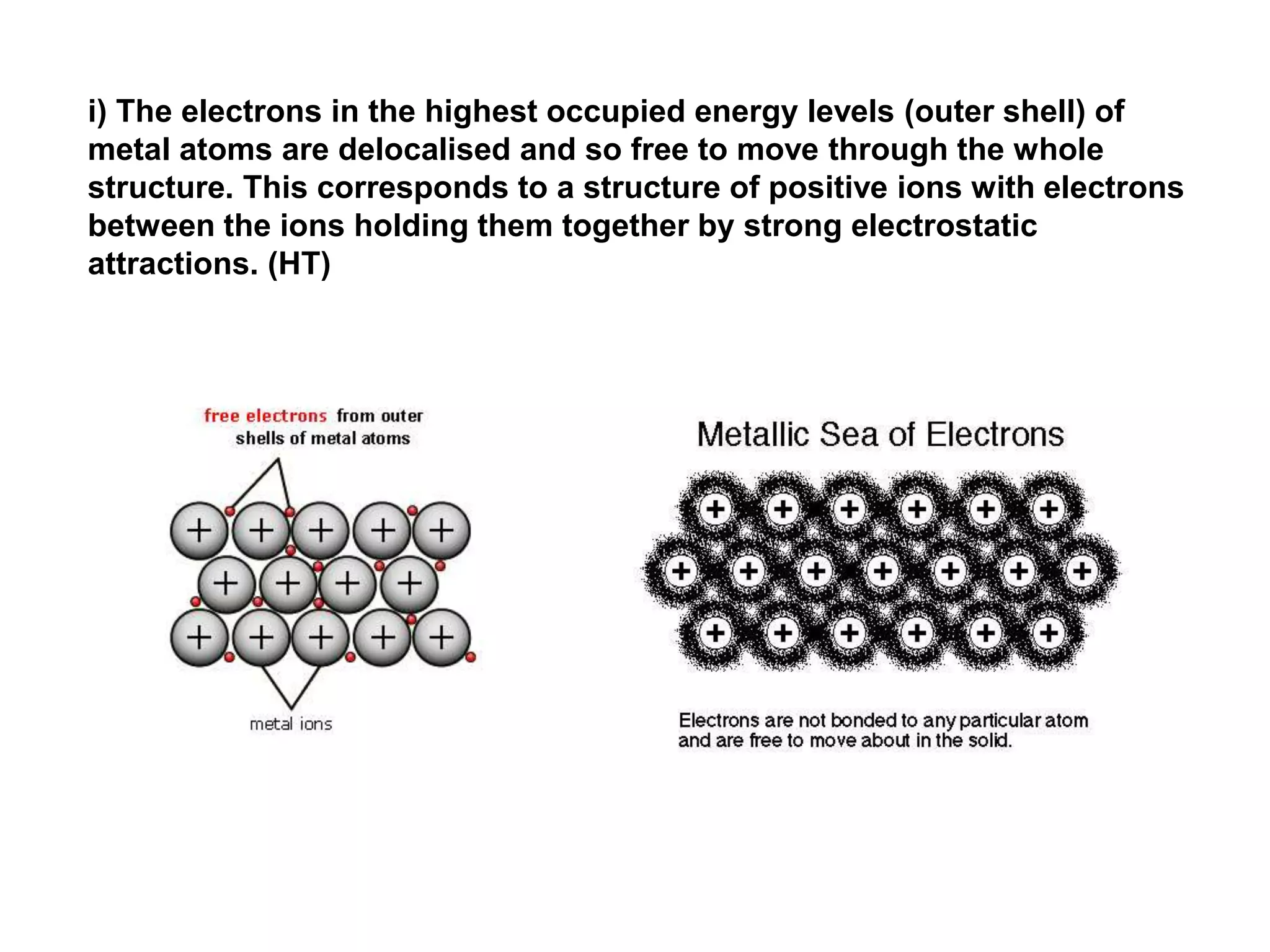
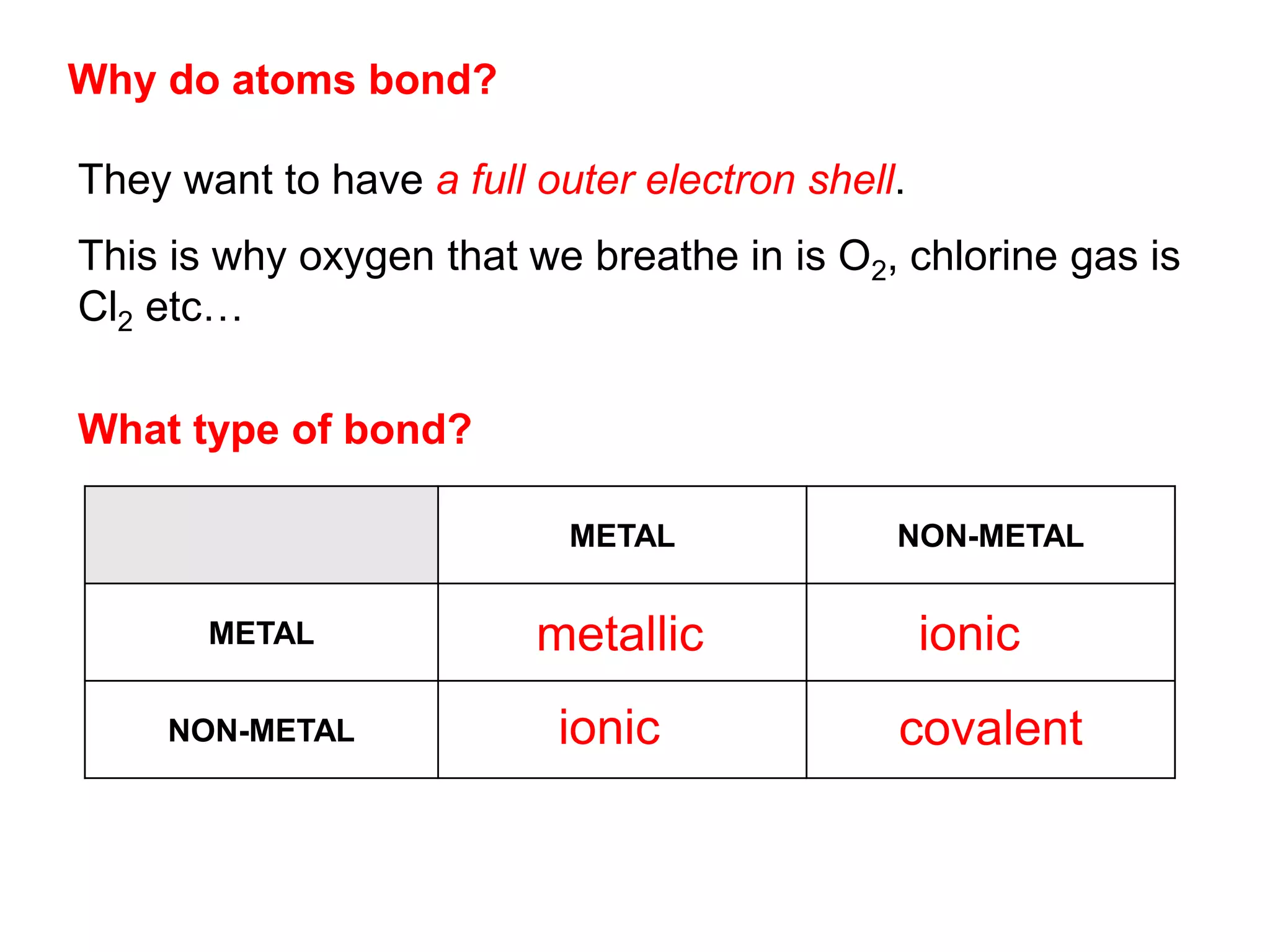
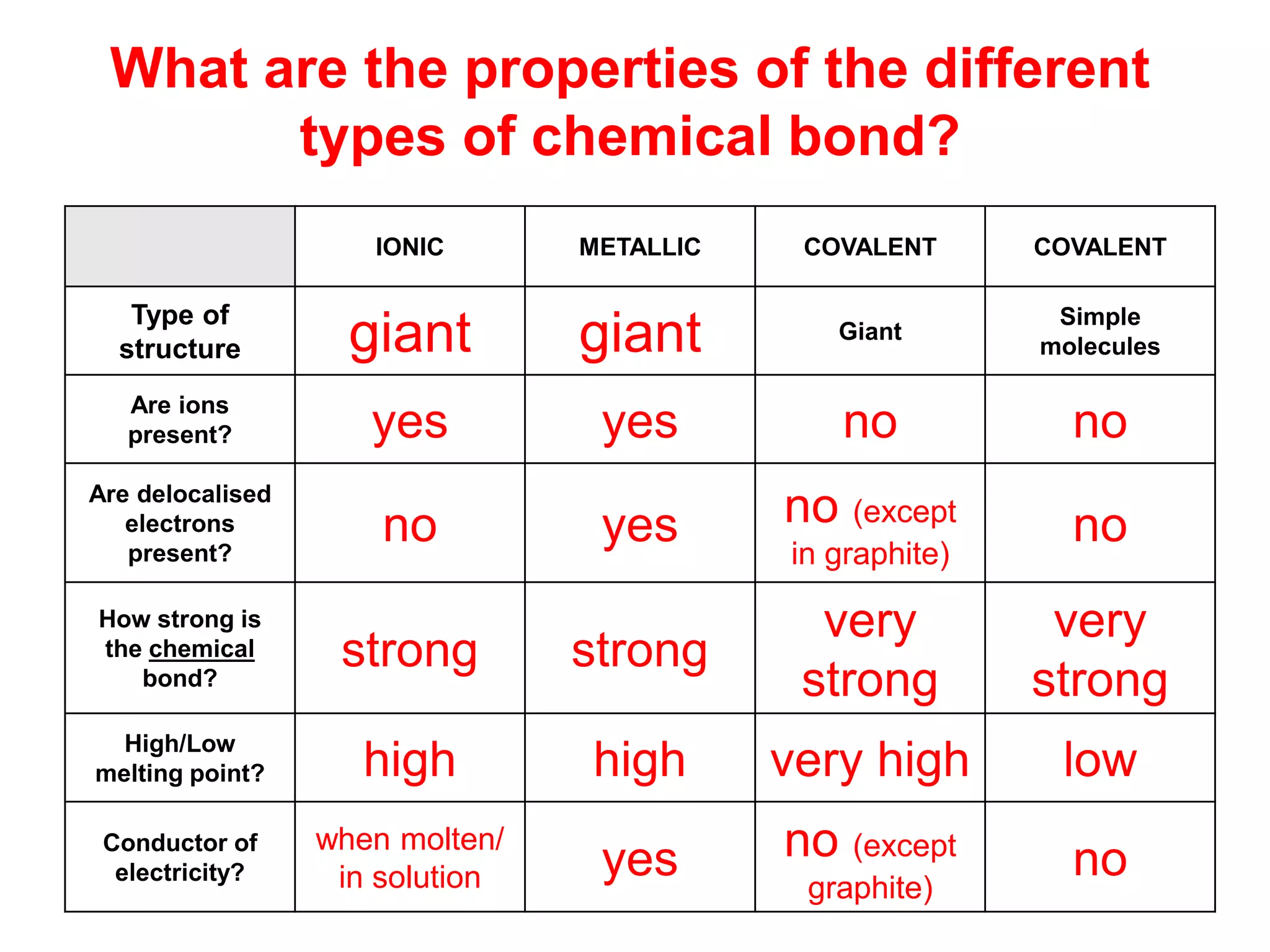
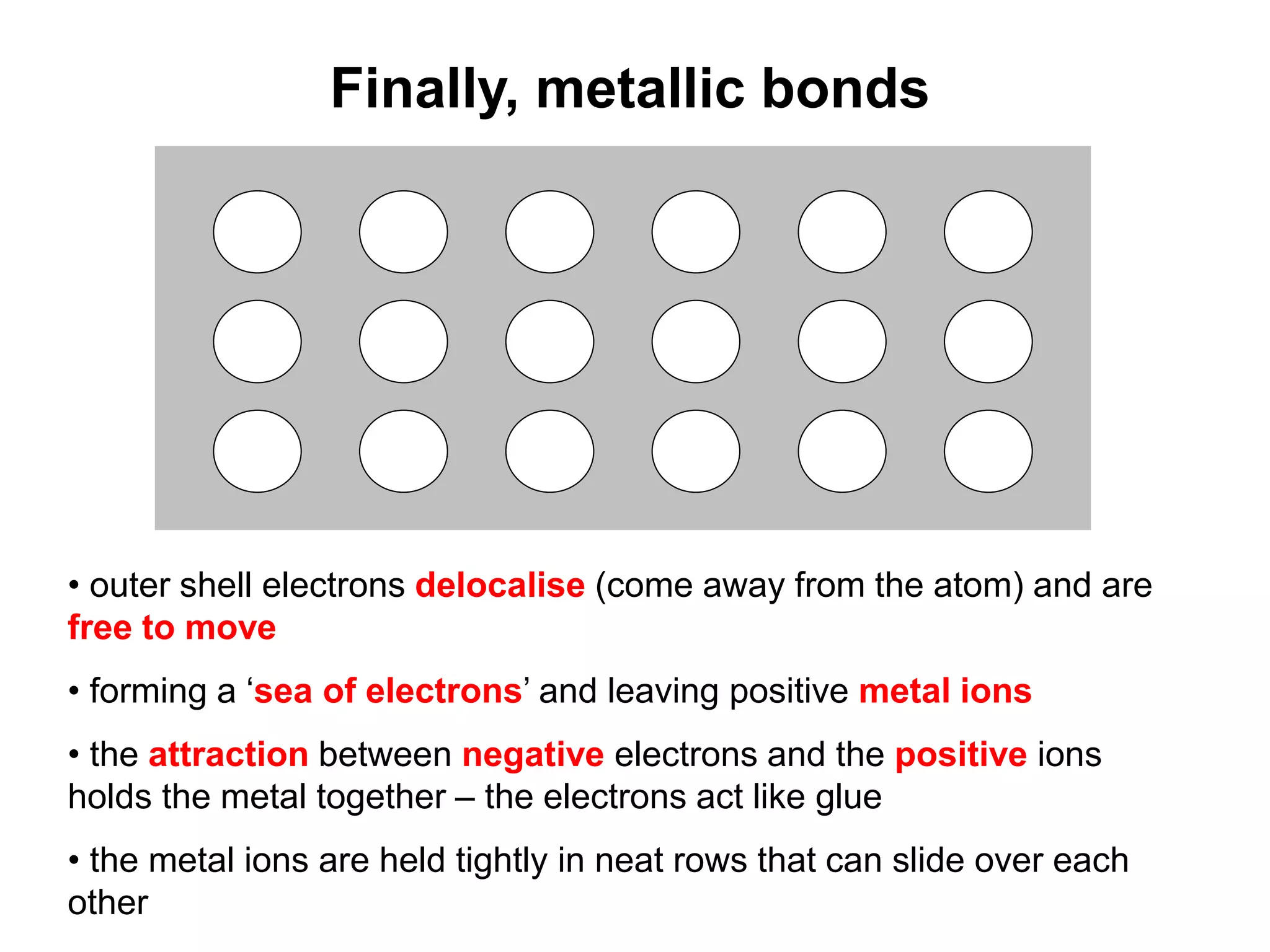
1. The document discusses different types of chemical bonding including ionic bonding, covalent bonding, and metallic bonding.
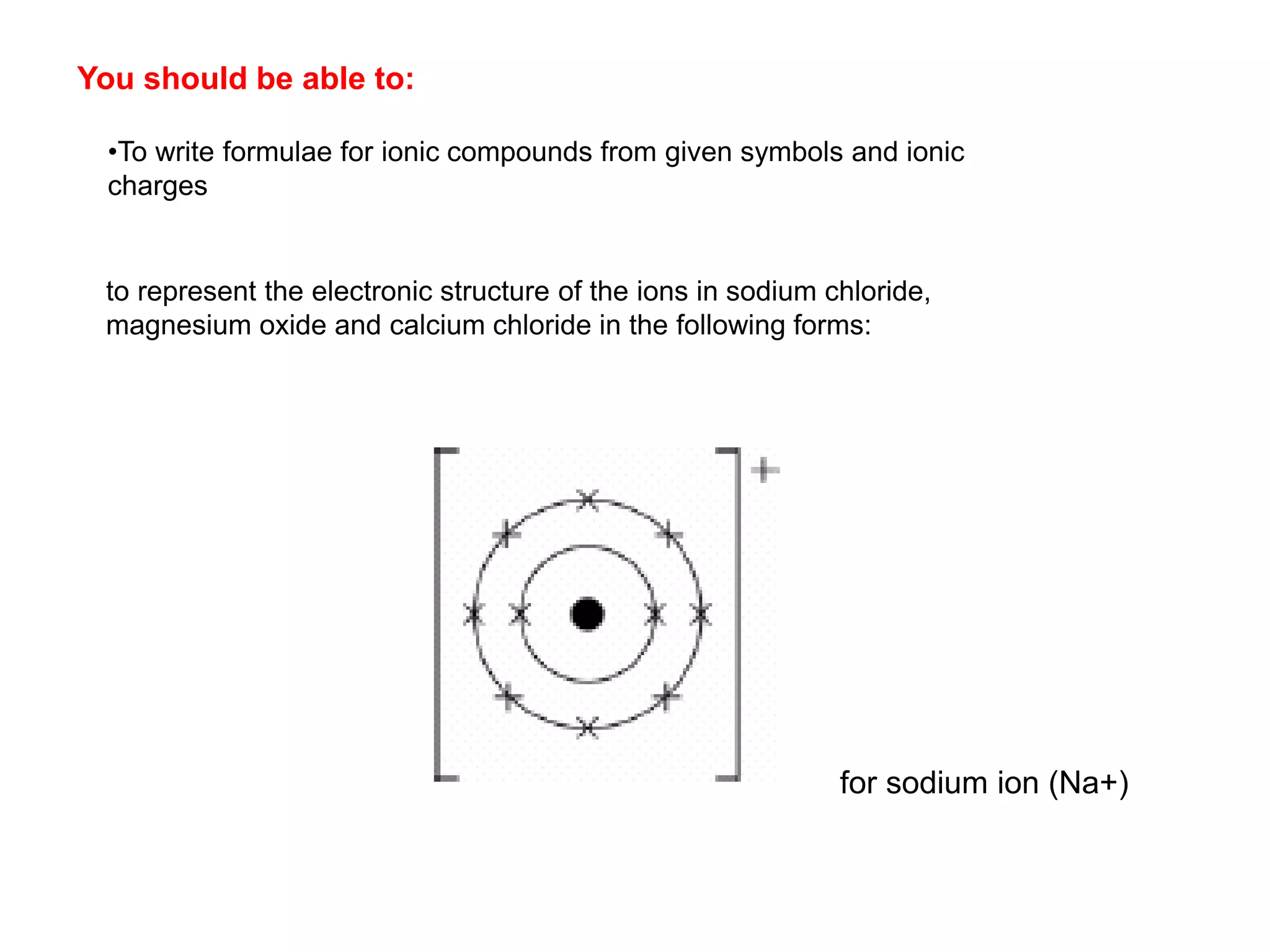
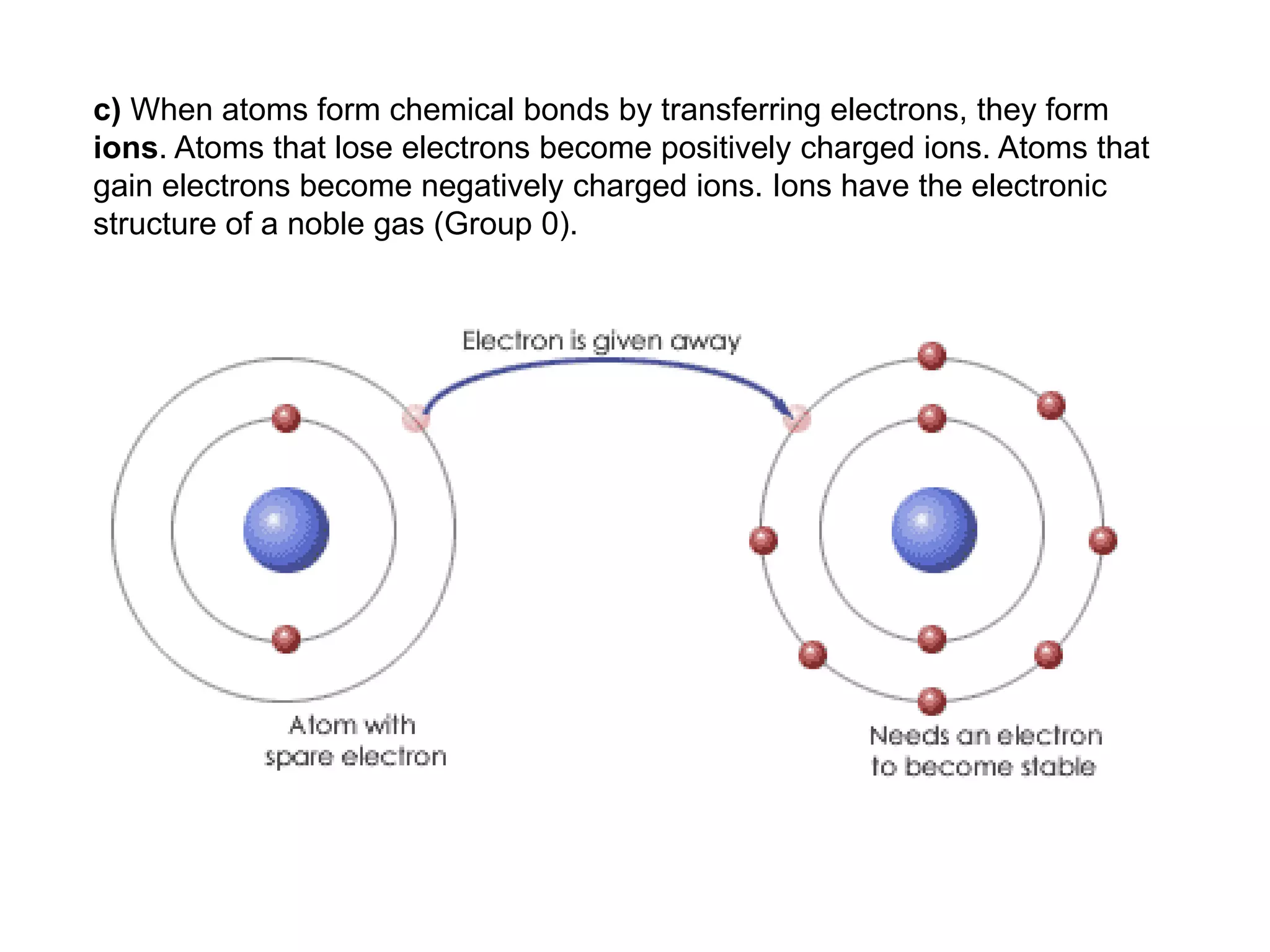
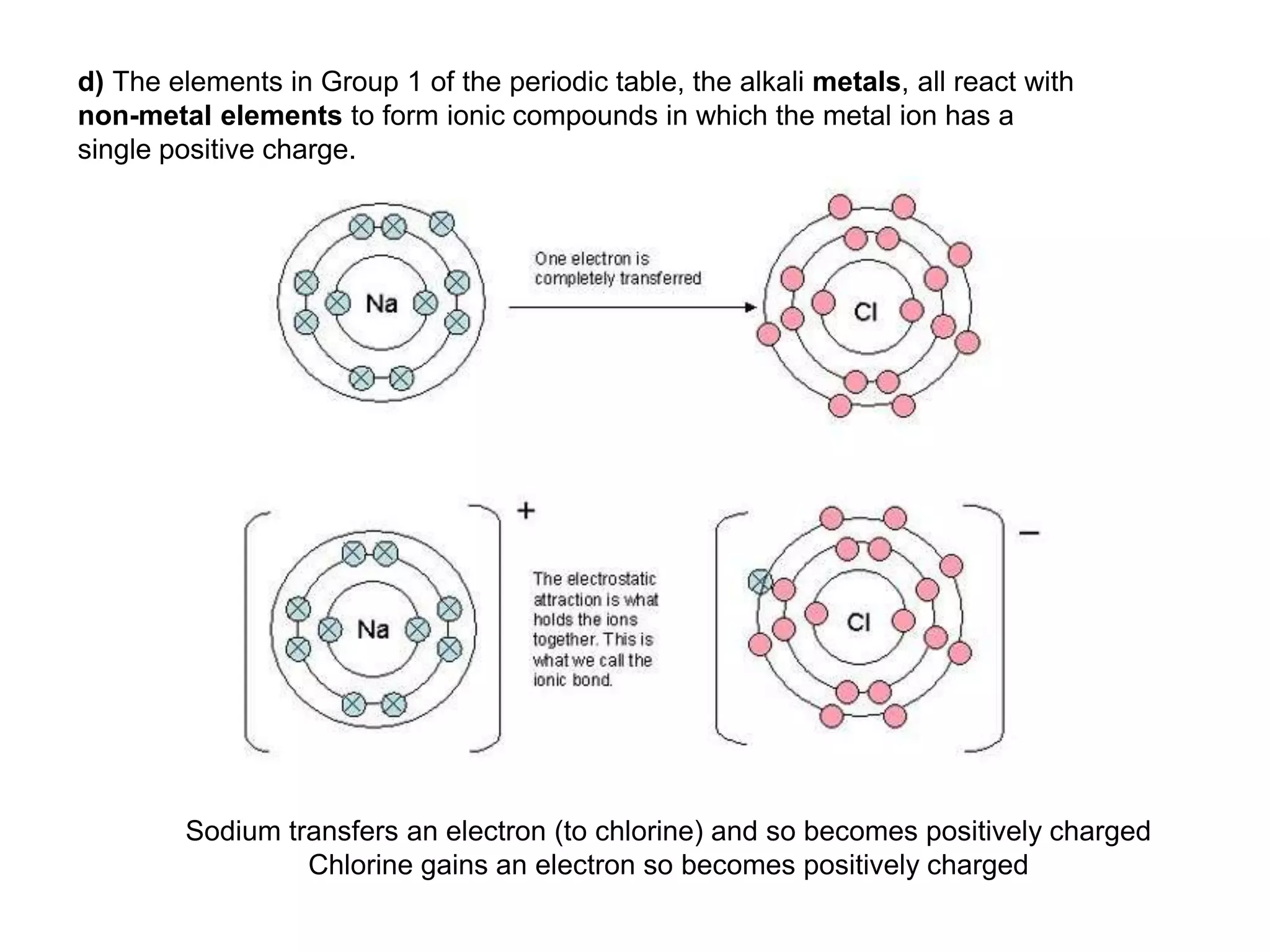
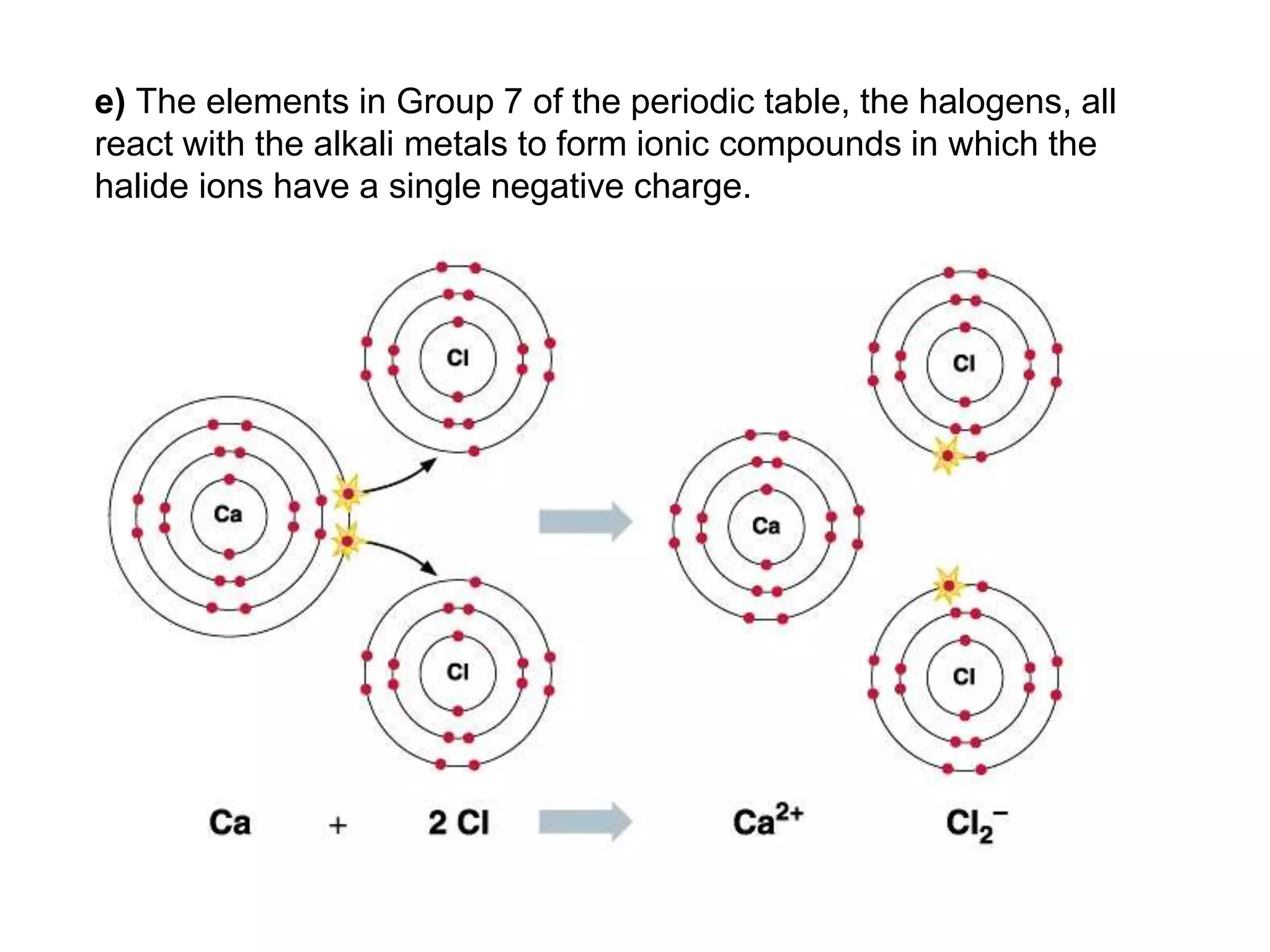
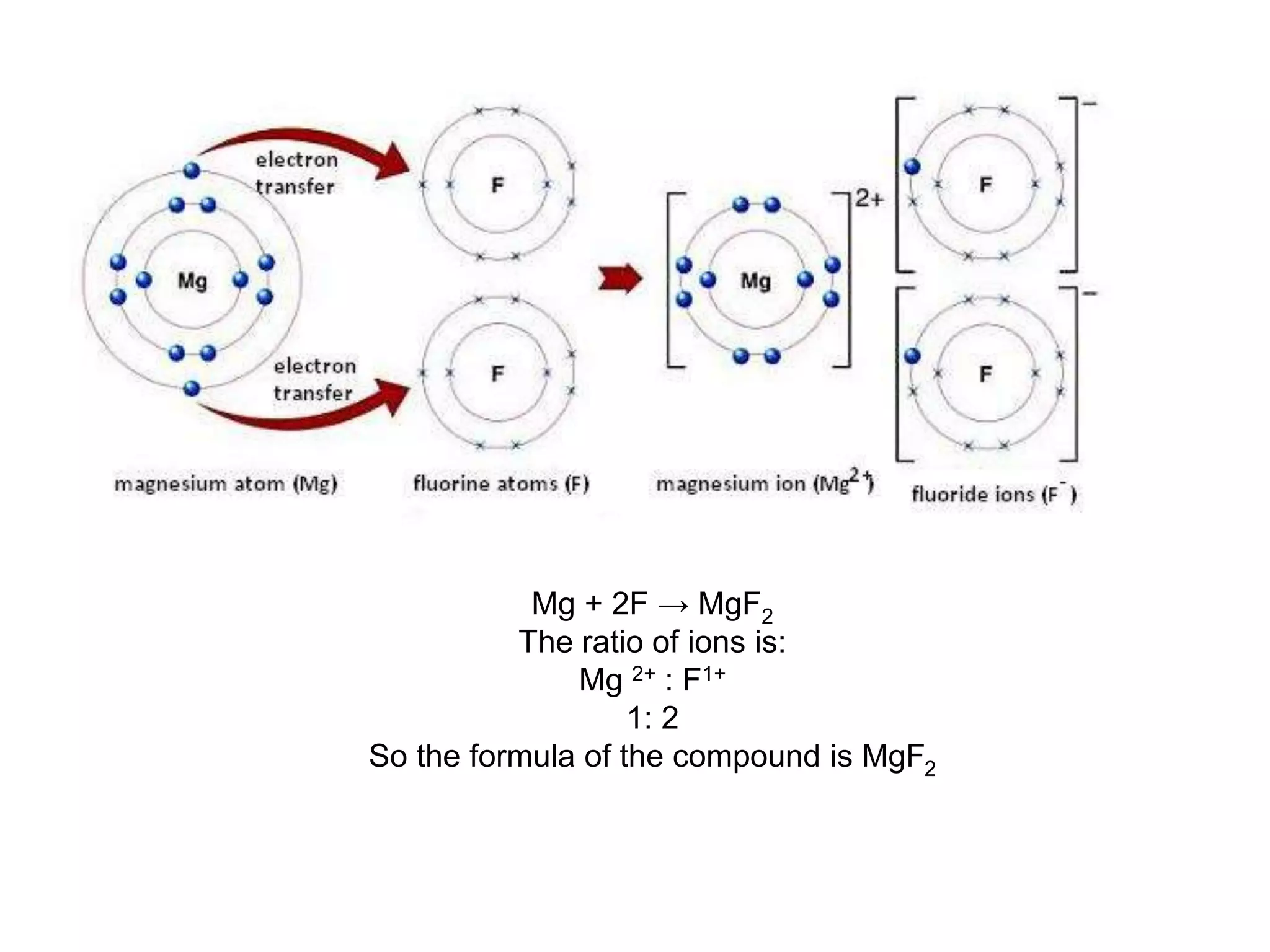
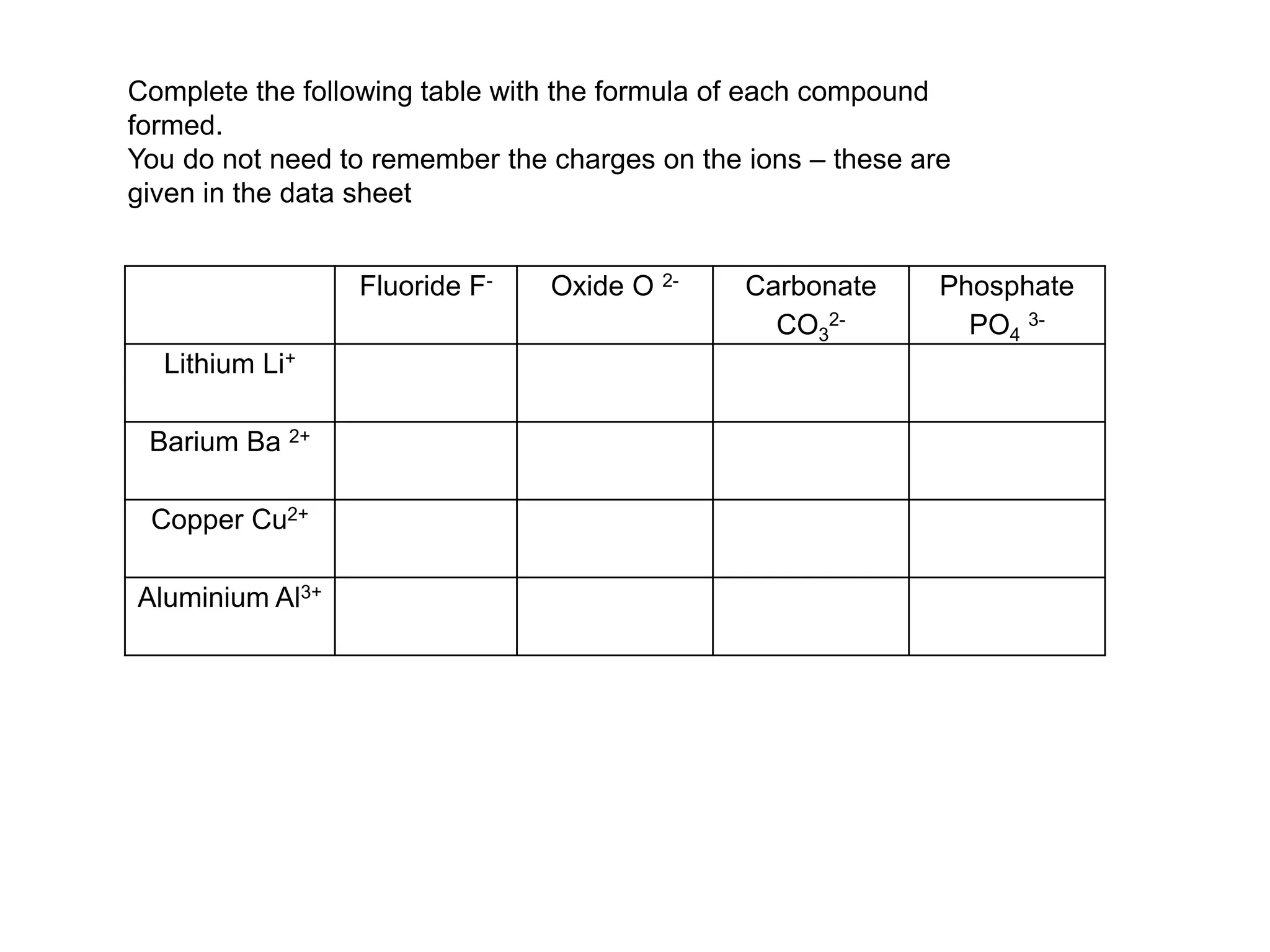
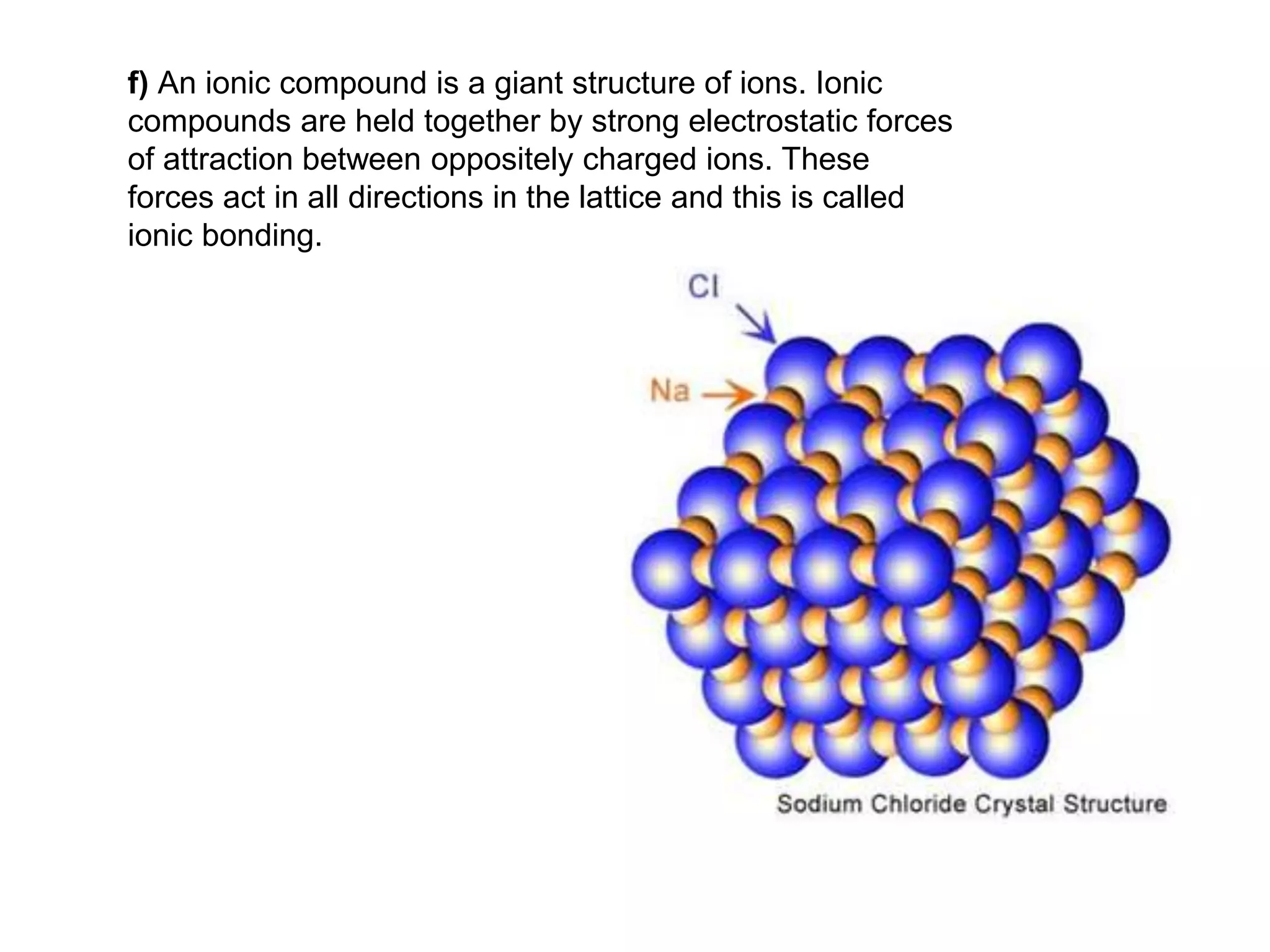
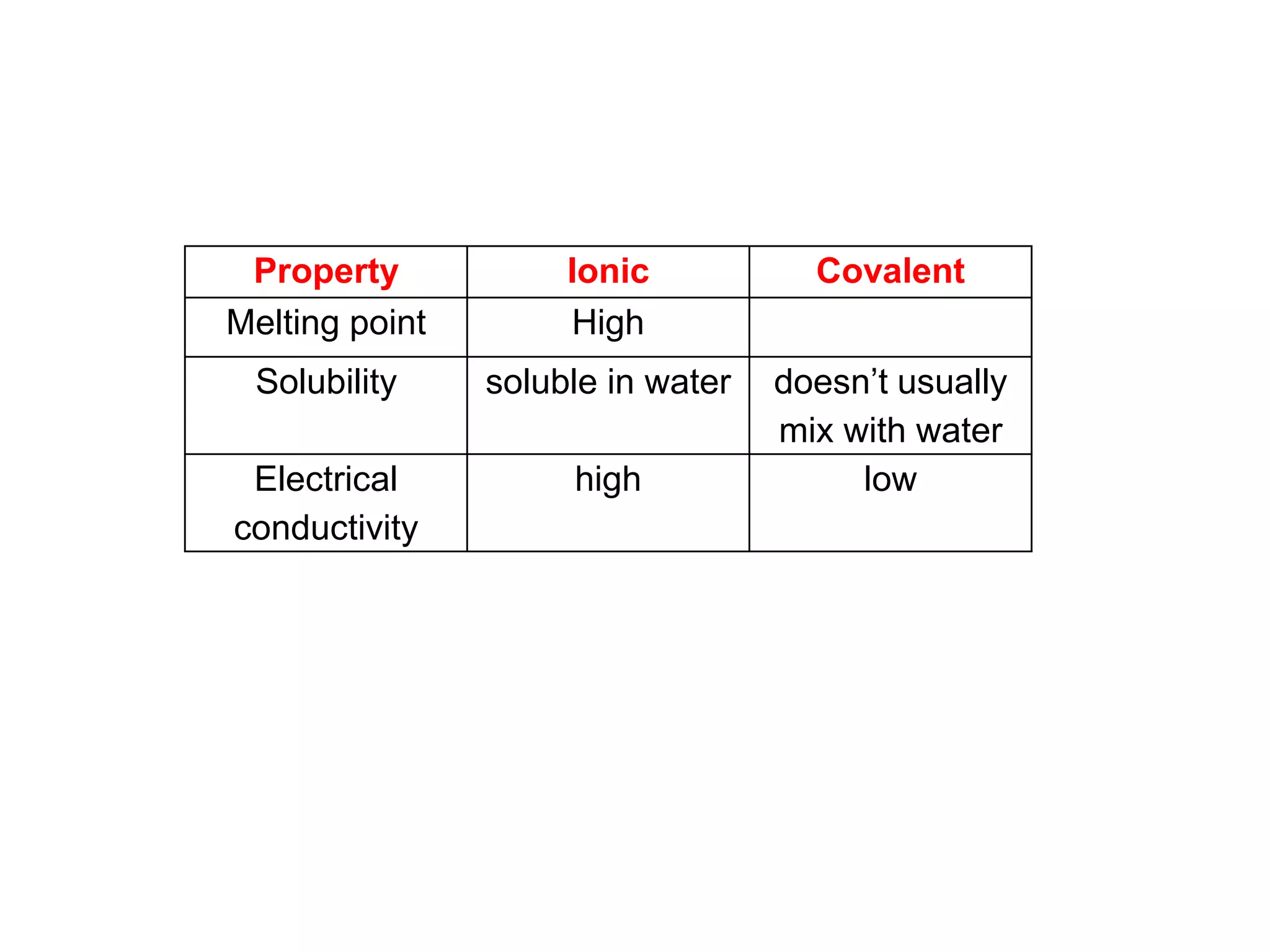
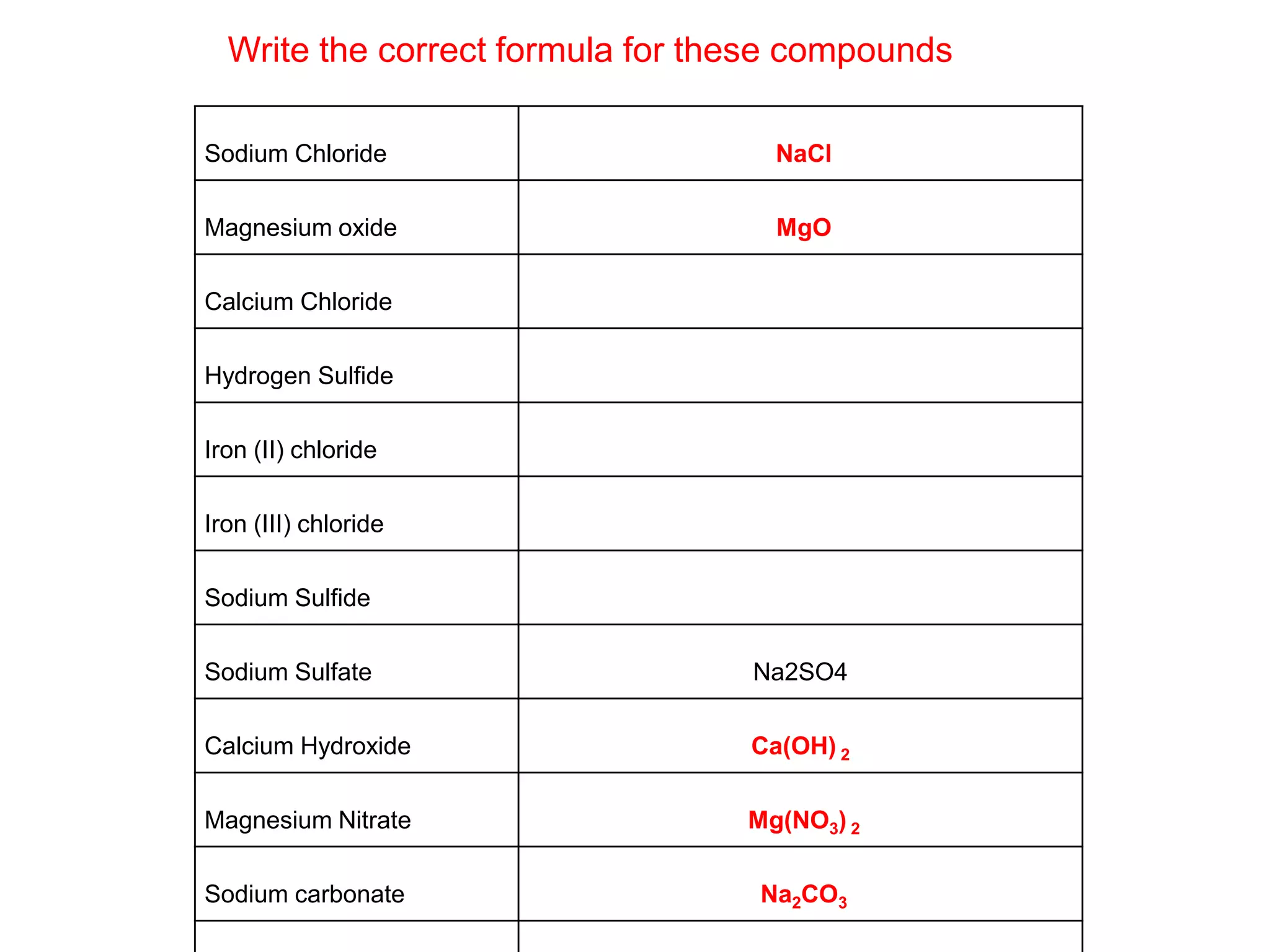
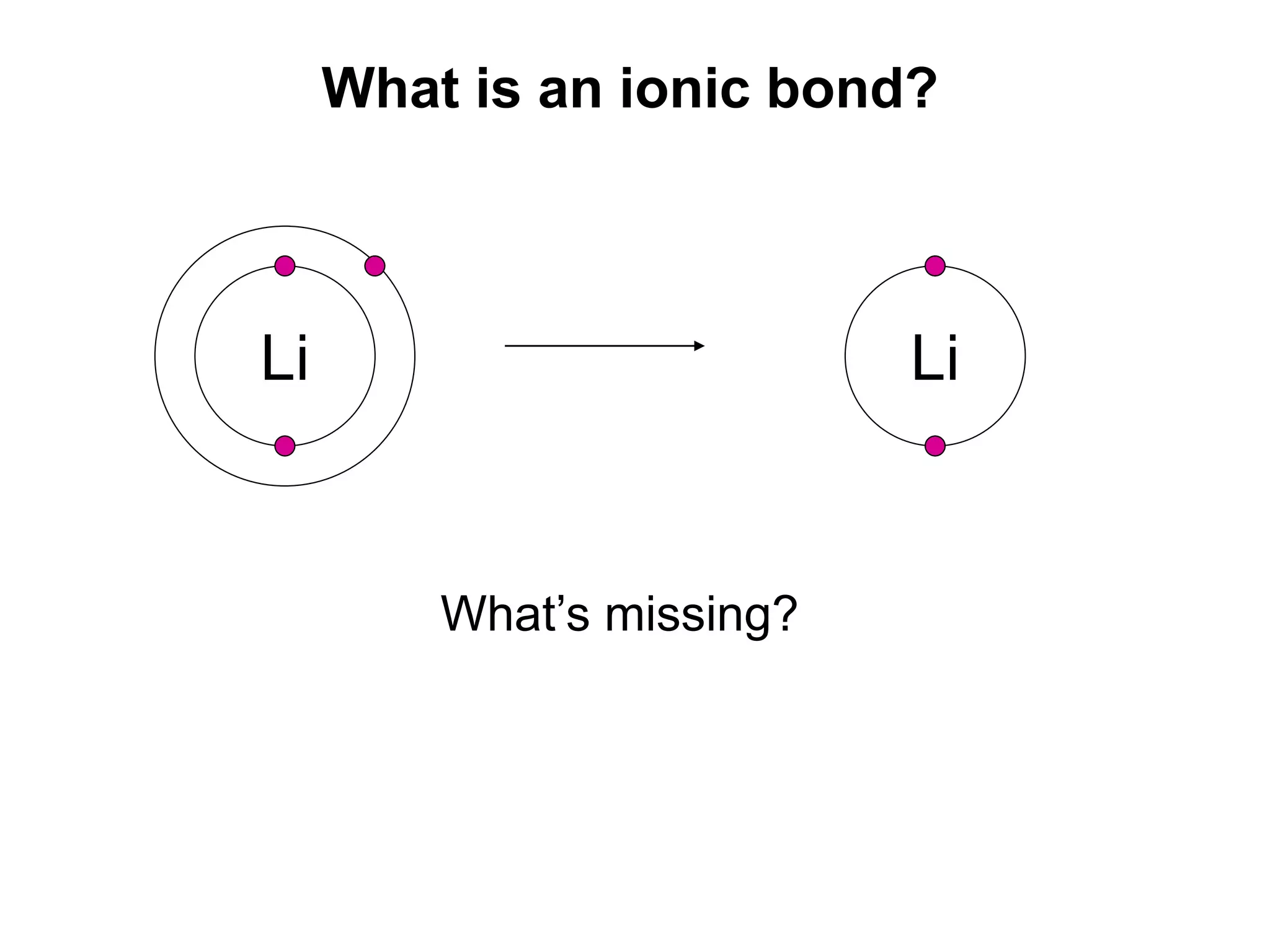
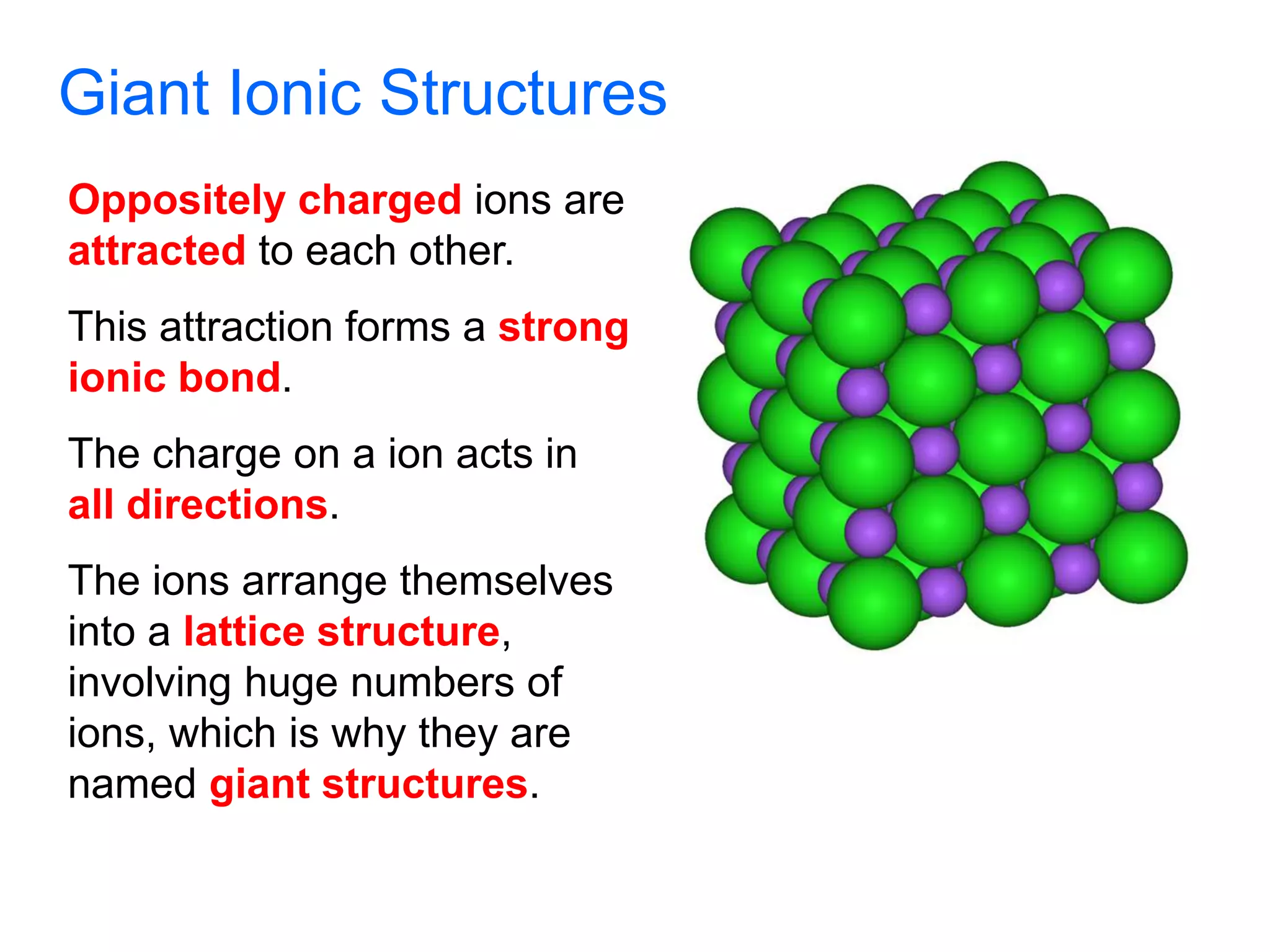
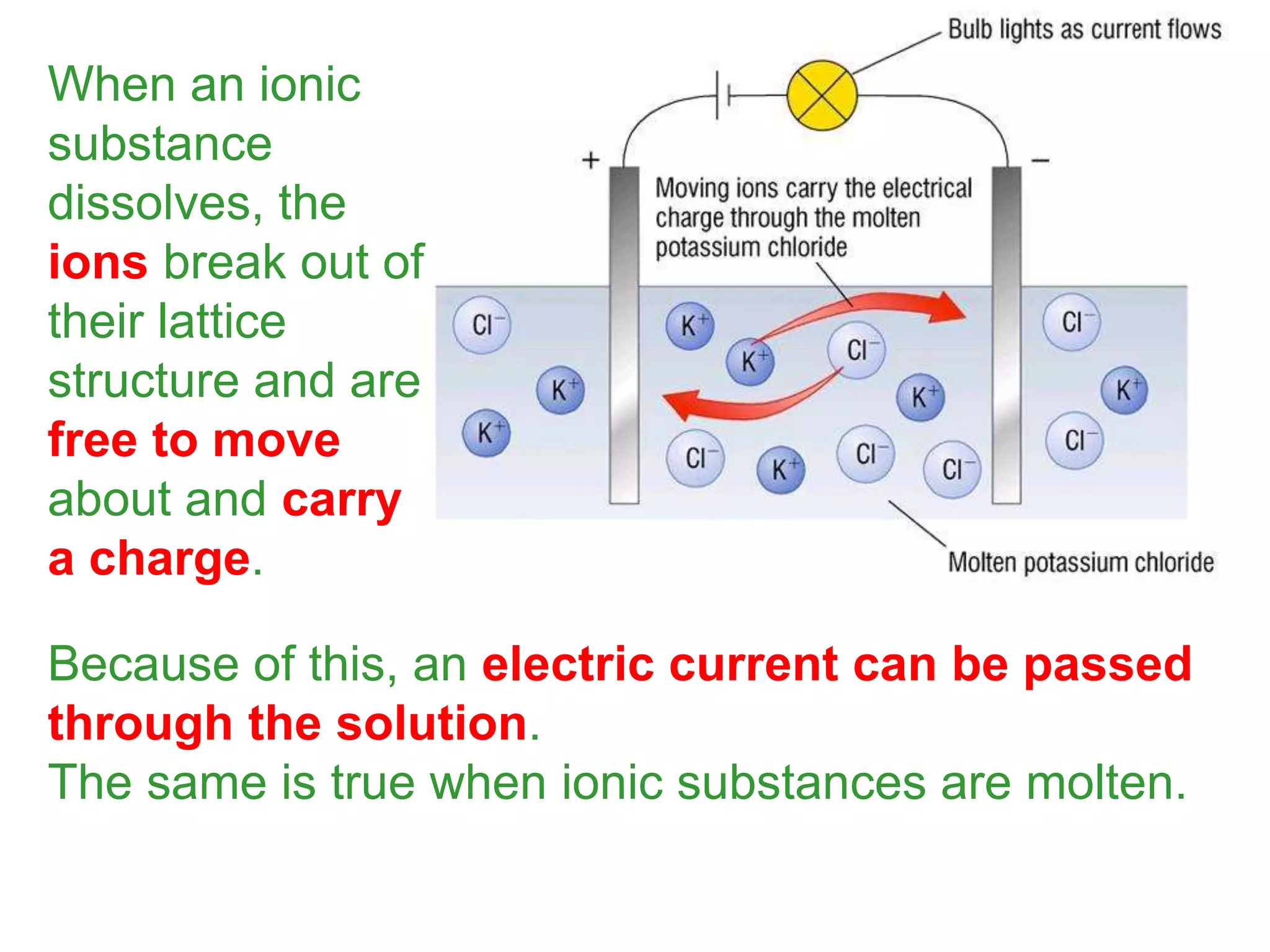
2. Ionic bonding involves the transfer of electrons between atoms to form ions with opposite charges that are attracted in a giant lattice structure.
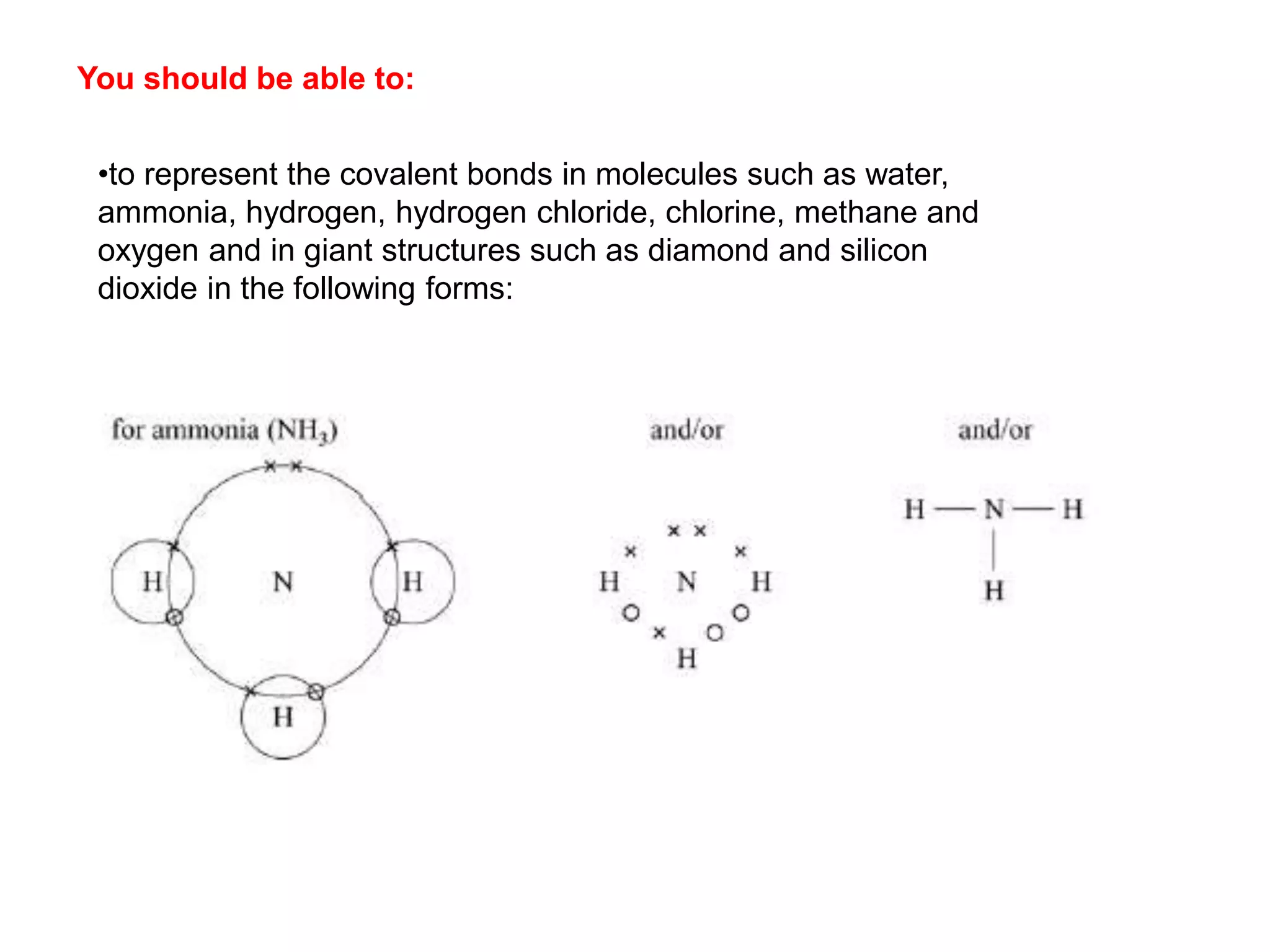
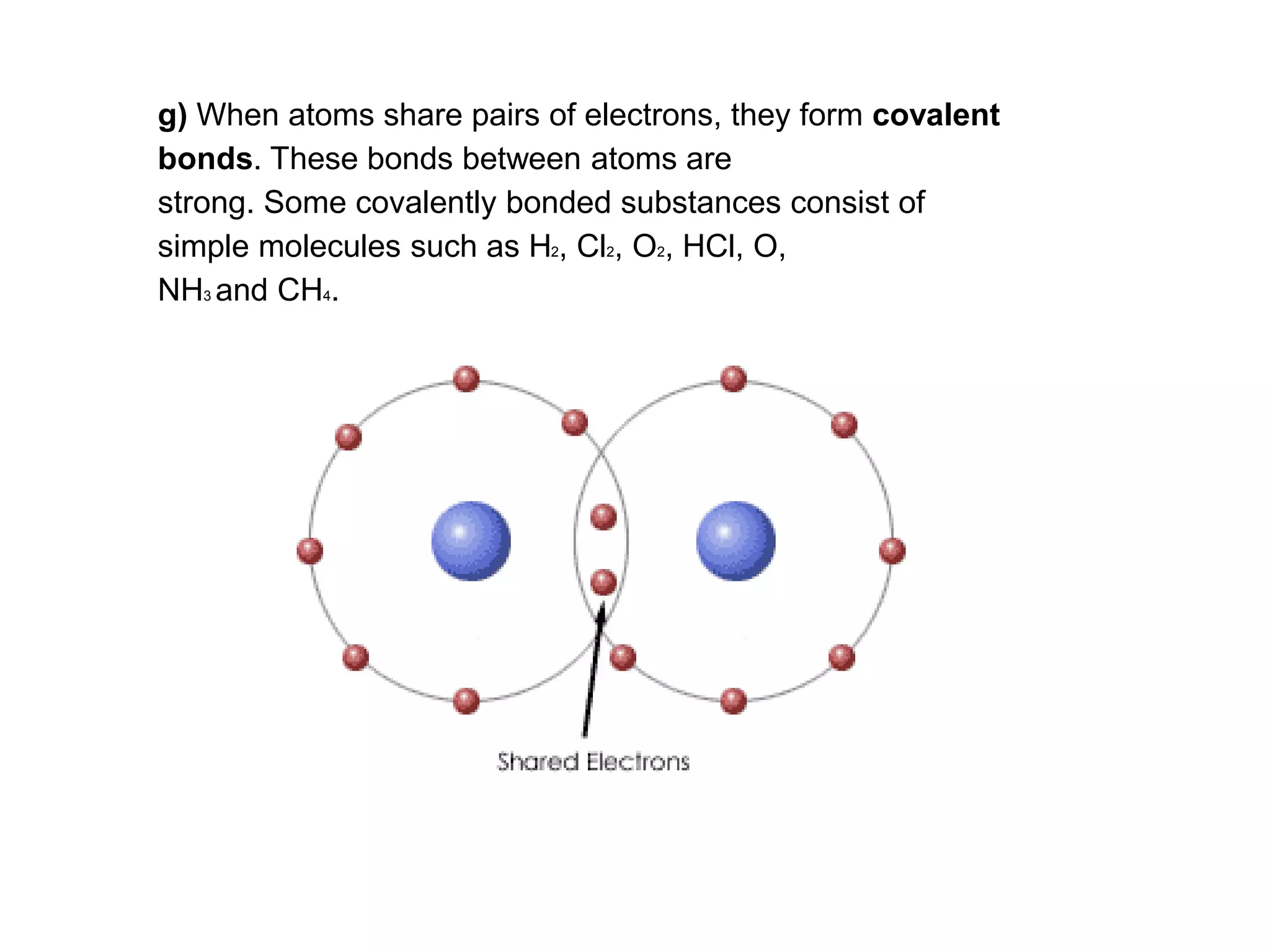
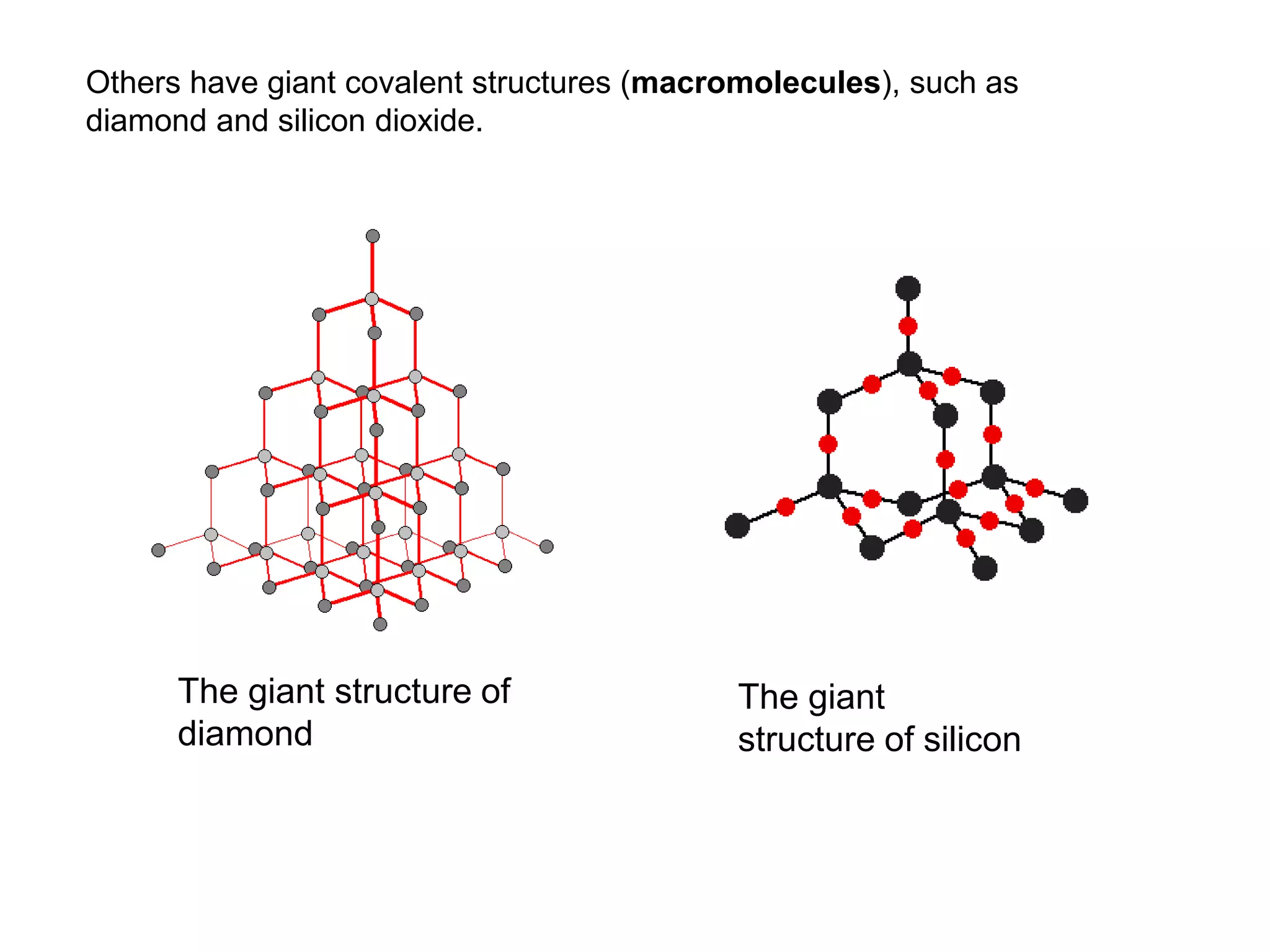
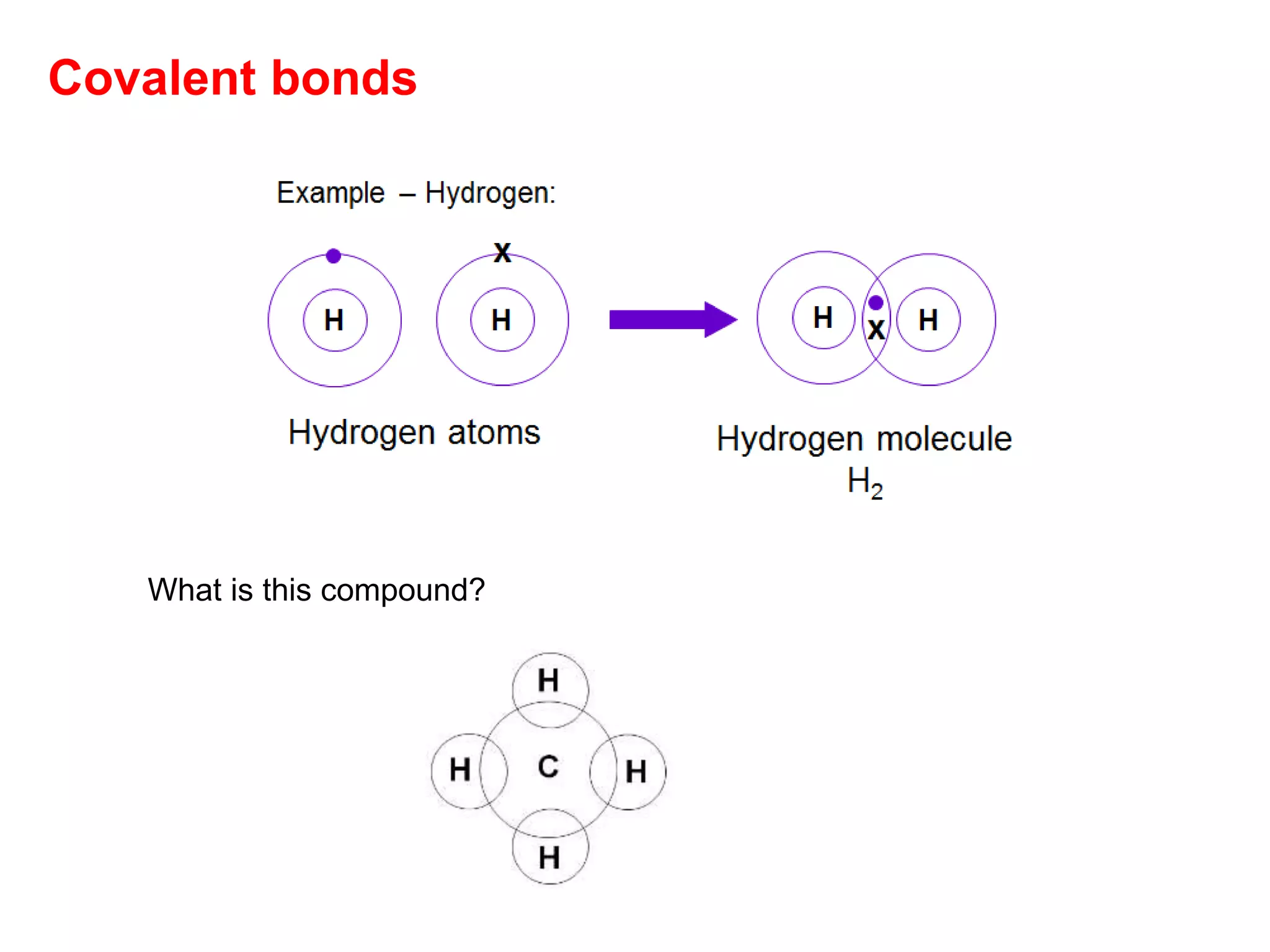
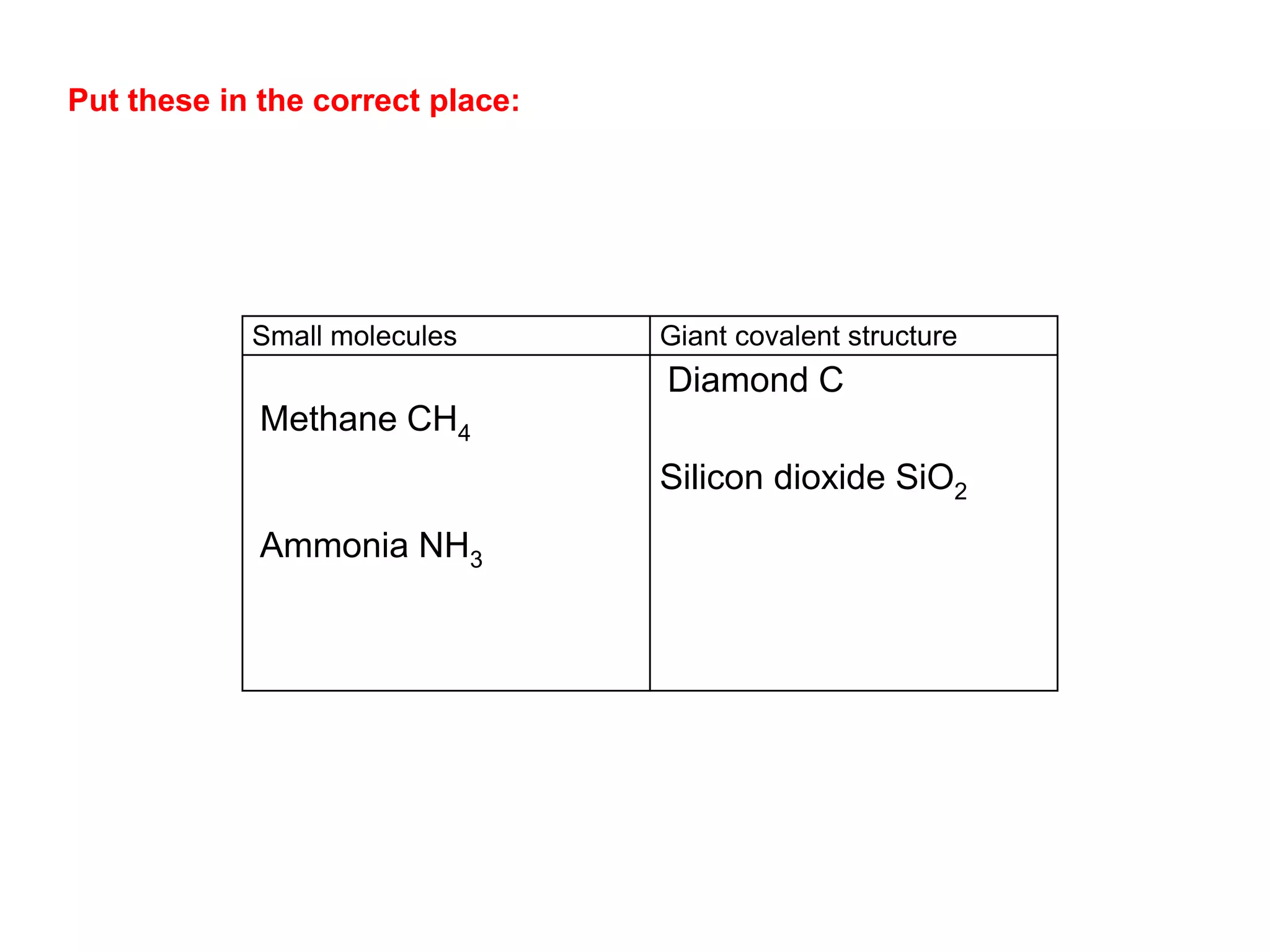
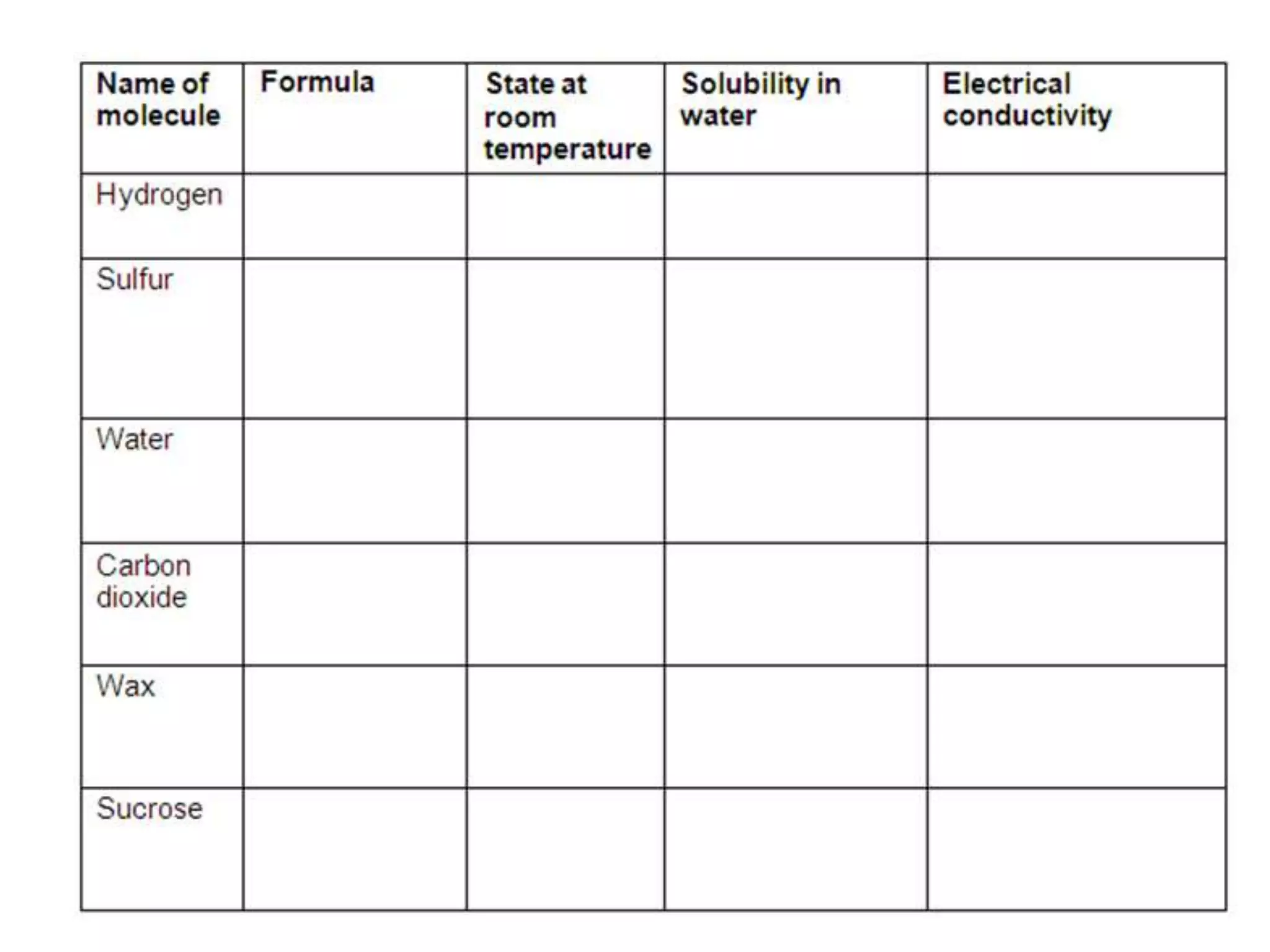
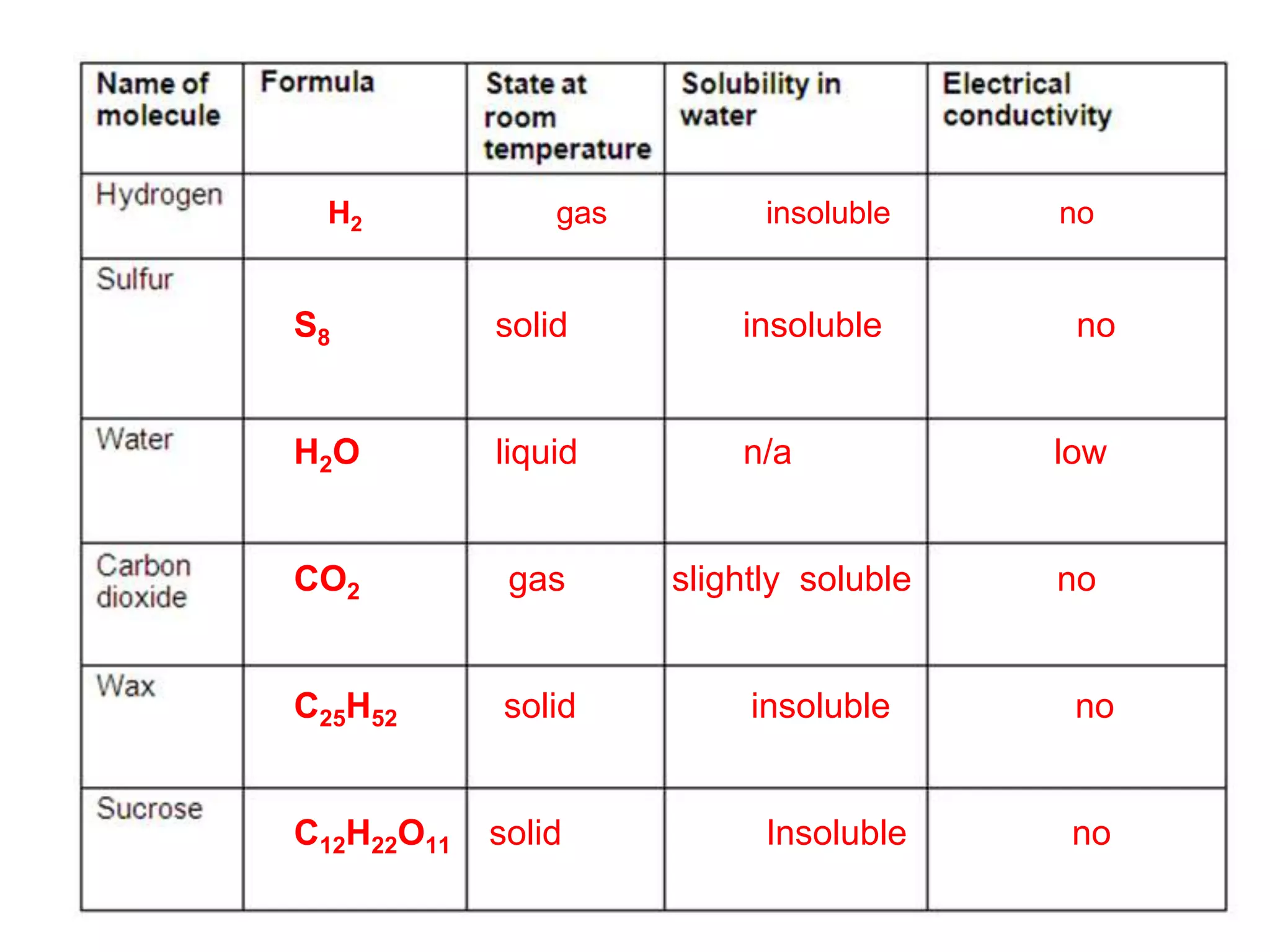
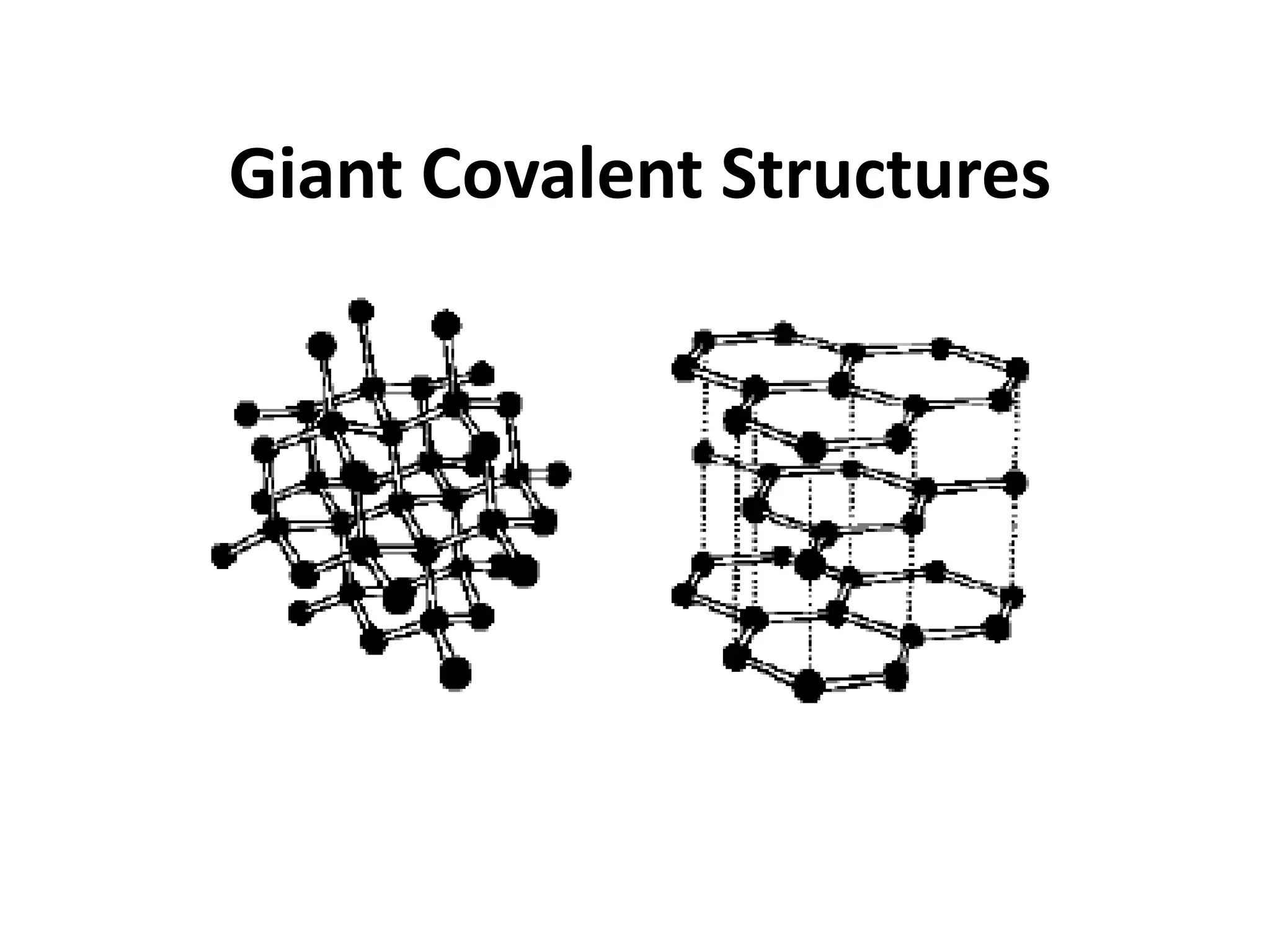
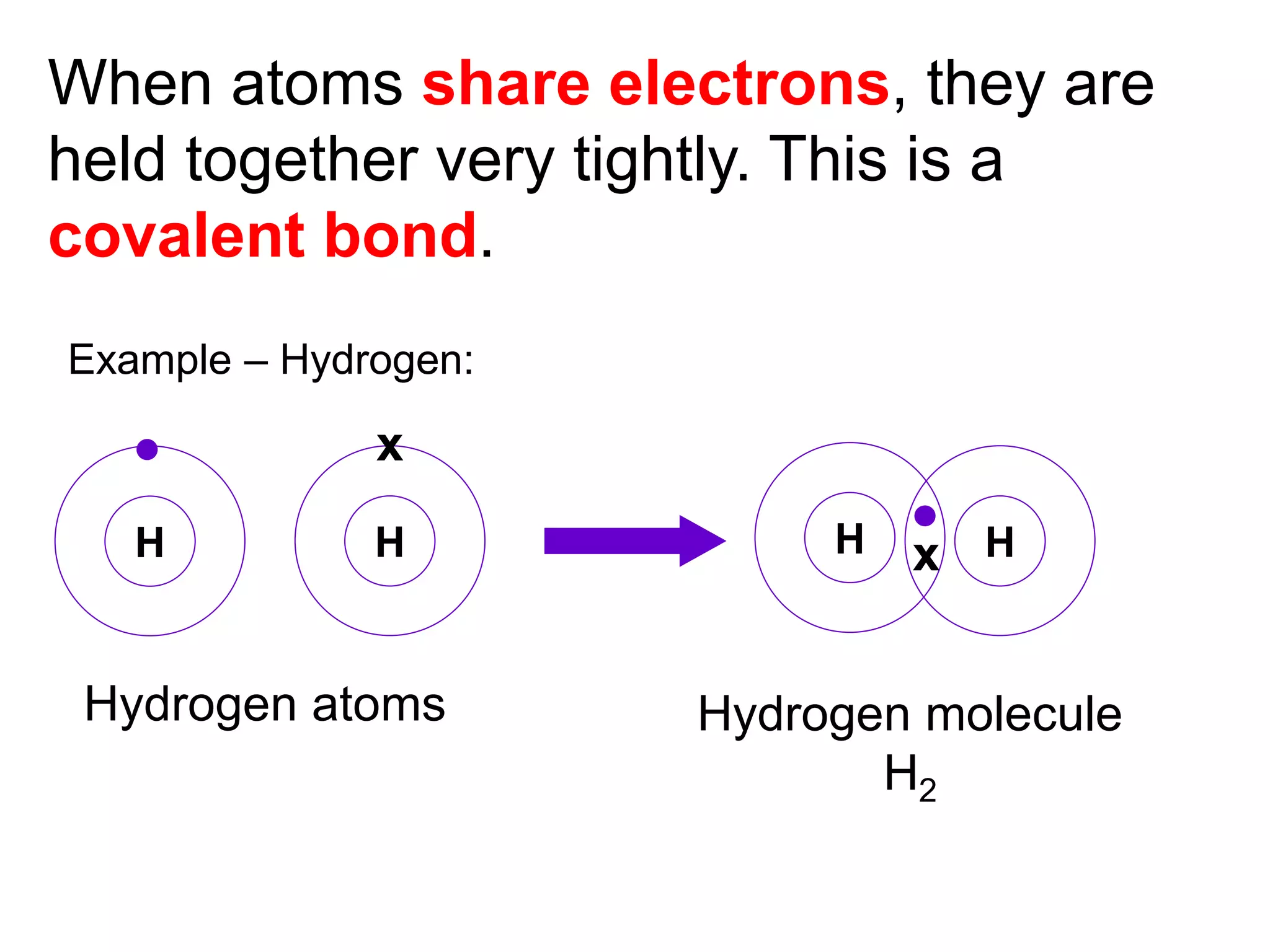
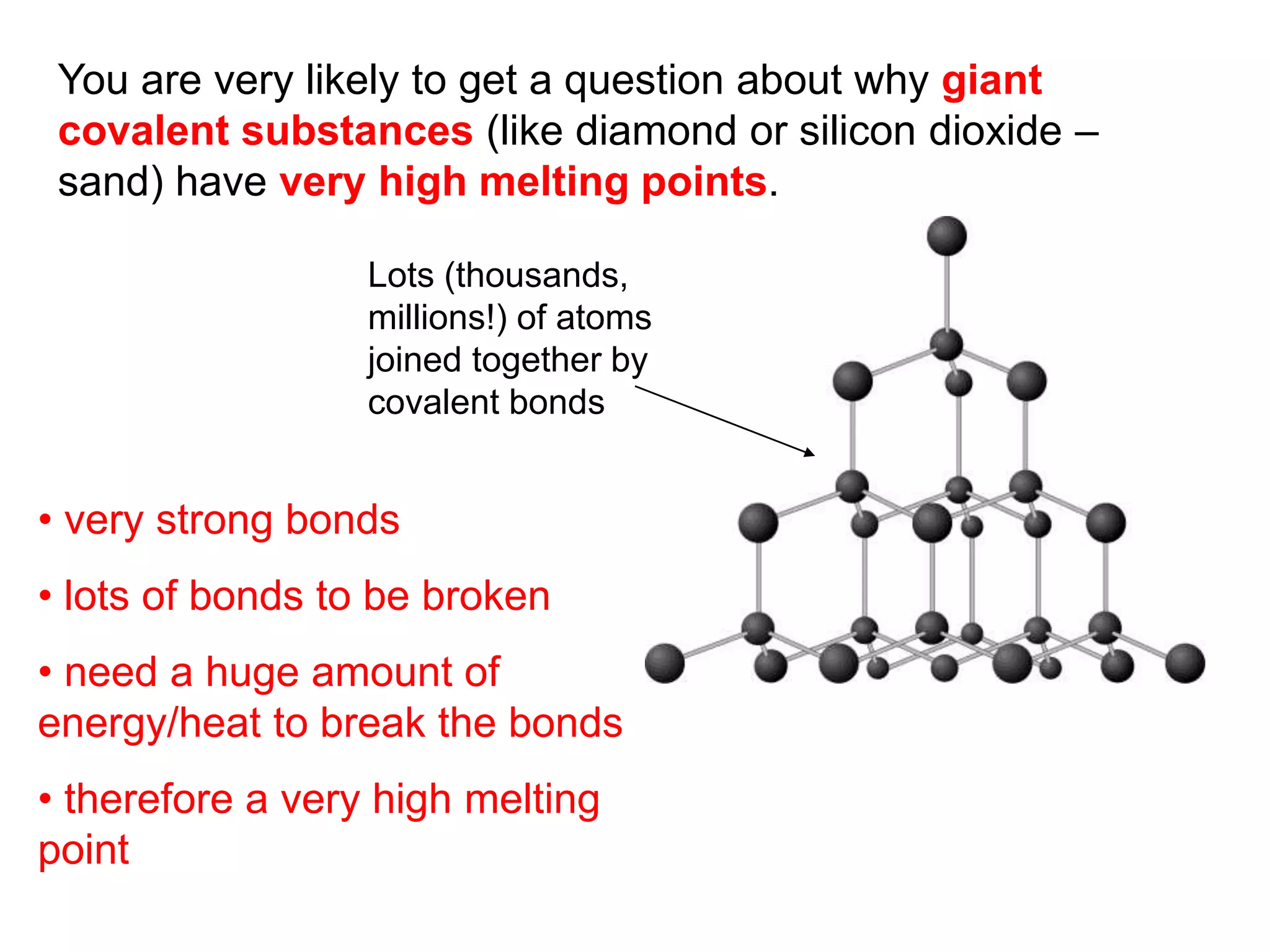
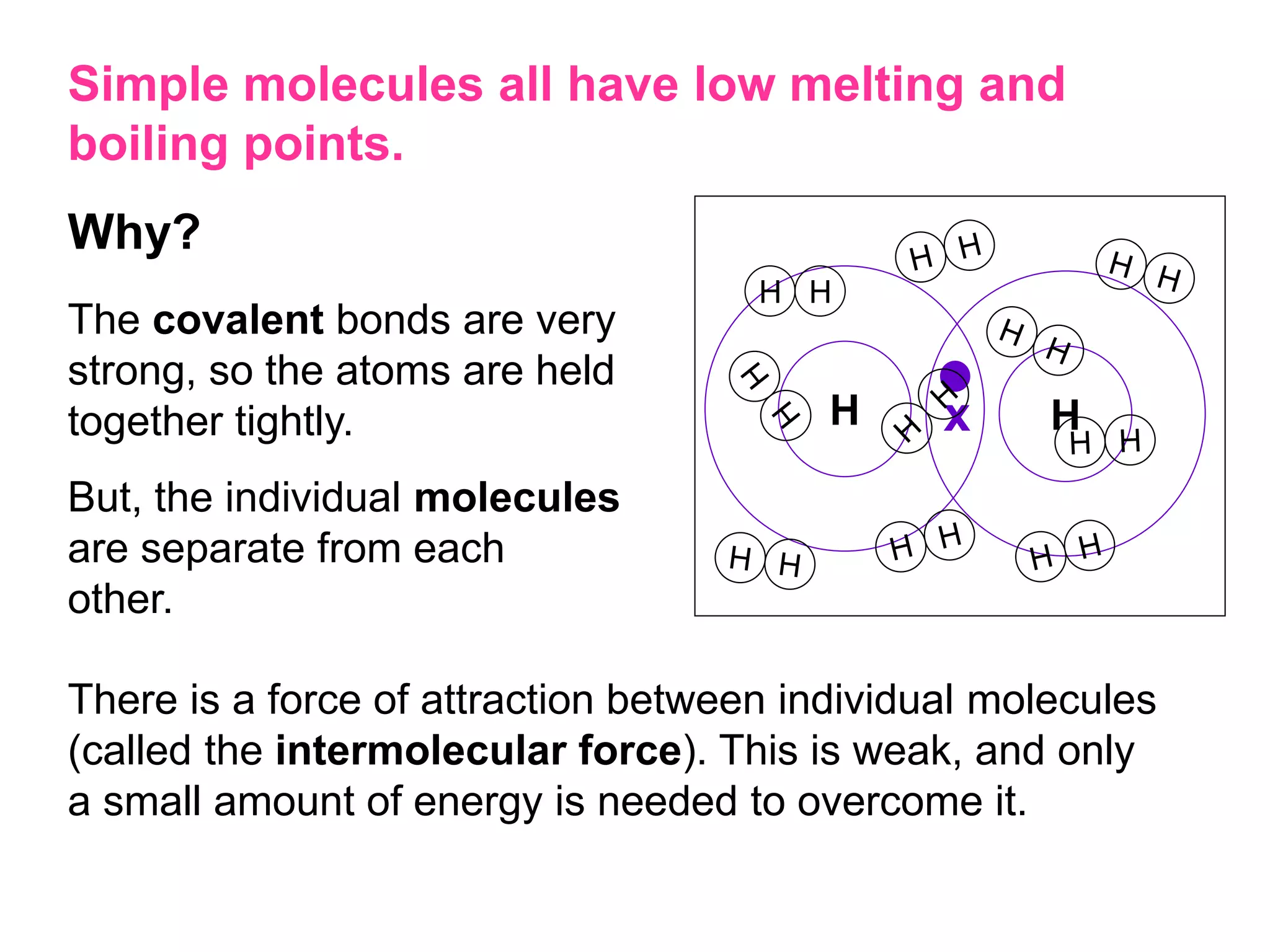
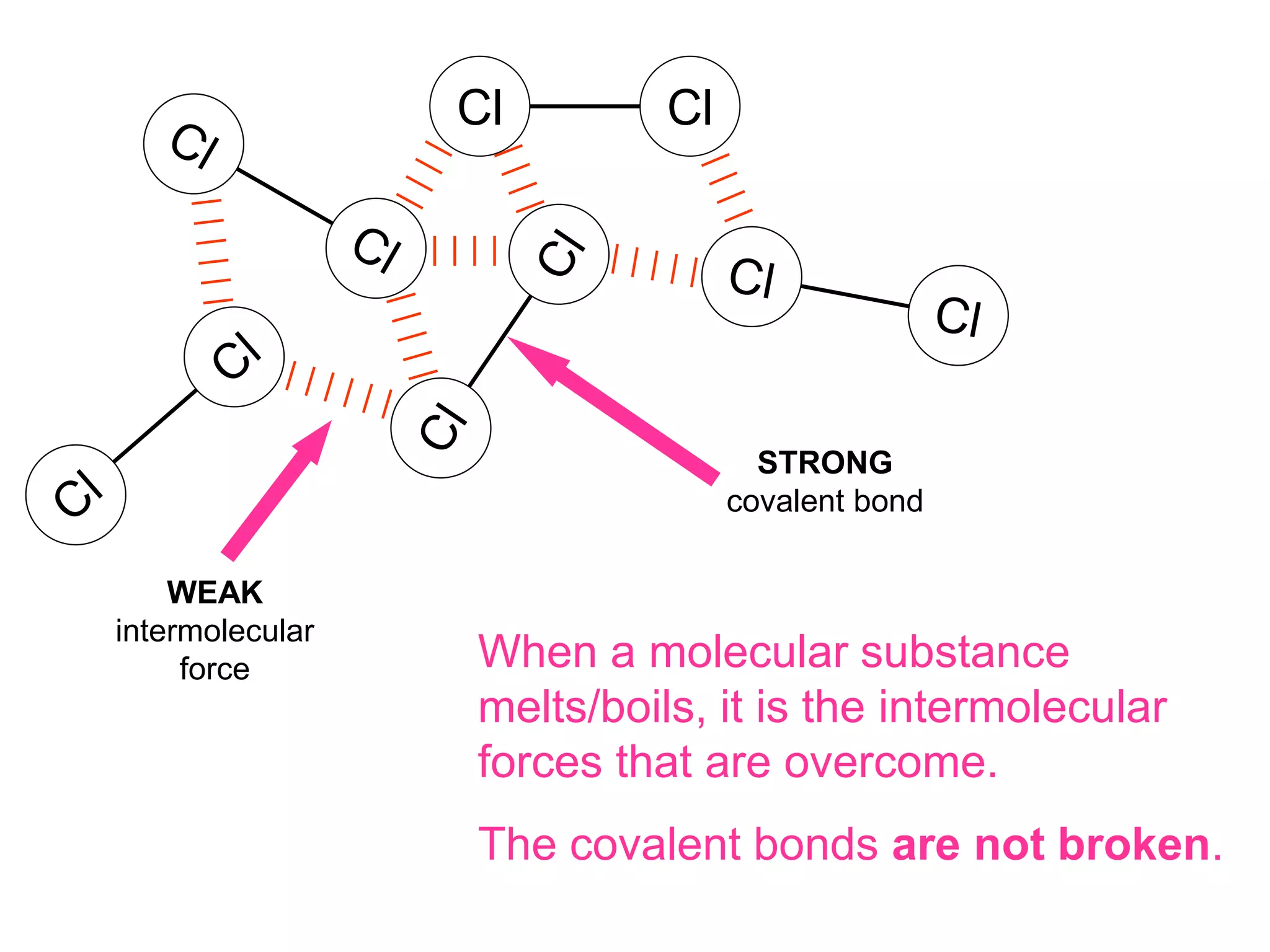
3. Covalent bonding can form either simple molecules held together by shared electron pairs or giant covalent structures with thousands of atoms bonded together.