Embed presentation
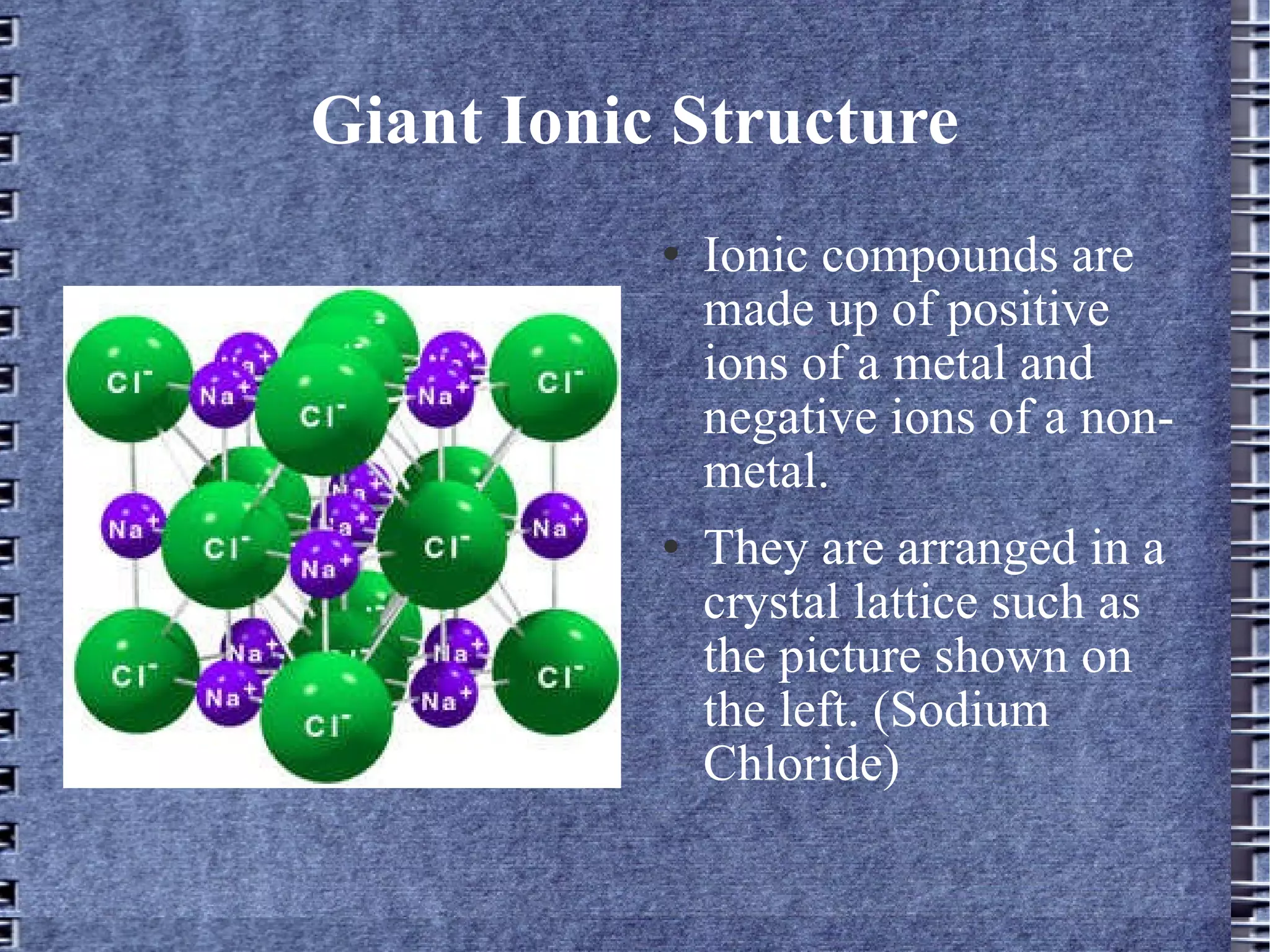




Ionic compounds are made up of positive metal ions and negative non-metal ions arranged in a crystal lattice. In ionic compounds like sodium chloride, the oppositely charged ions are held together by strong electrostatic forces of attraction. Potassium oxide and lithium oxide are examples where the metal ions (potassium and lithium) outnumber the oxygen ions in a ratio of about 1:2, as represented by their chemical formulas K2O and Li2O.




