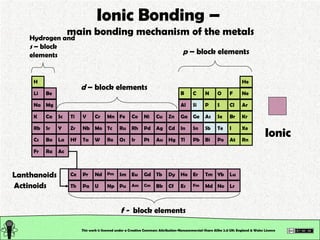
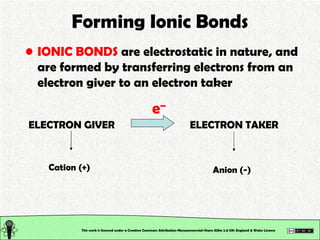
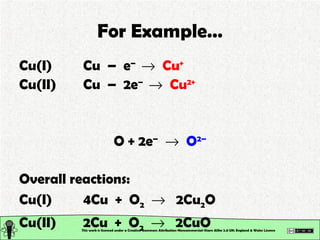
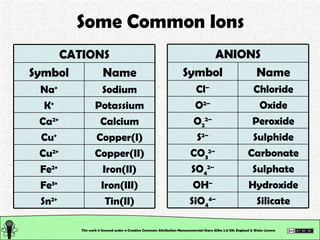
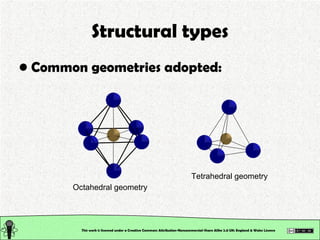
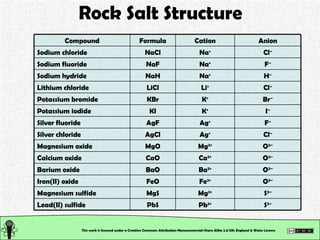
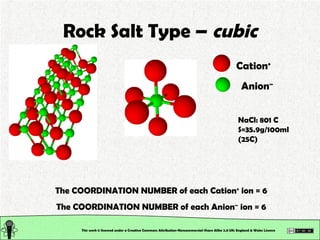
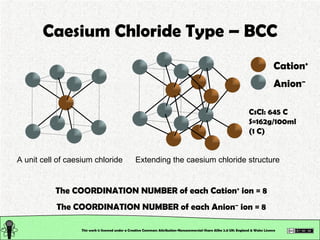
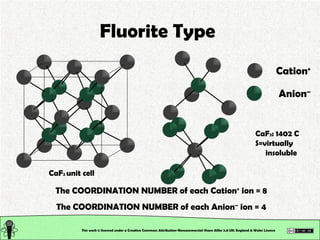
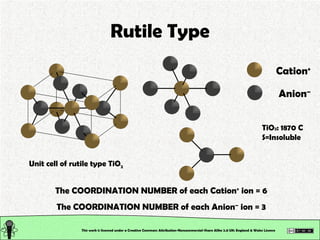
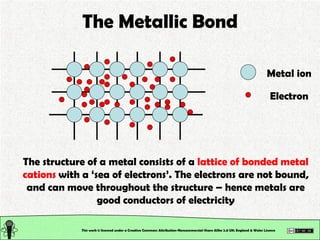
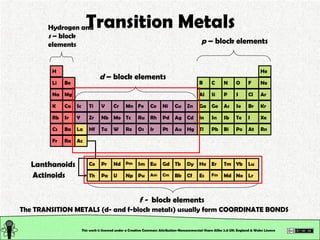
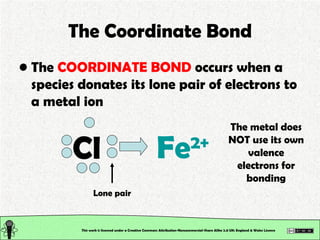
The document explains various types of chemical bonding, focusing on ionic, metallic, and coordinate bonds. It details the nature of ionic bonds, their formation through electron transfer, and provides examples of common ions and structural geometries in ionic compounds. Additionally, it covers metallic bonding, emphasizing the sharing of electrons among metal atoms, and introduces coordinate bonding through ligands donating lone pairs to metal ions.