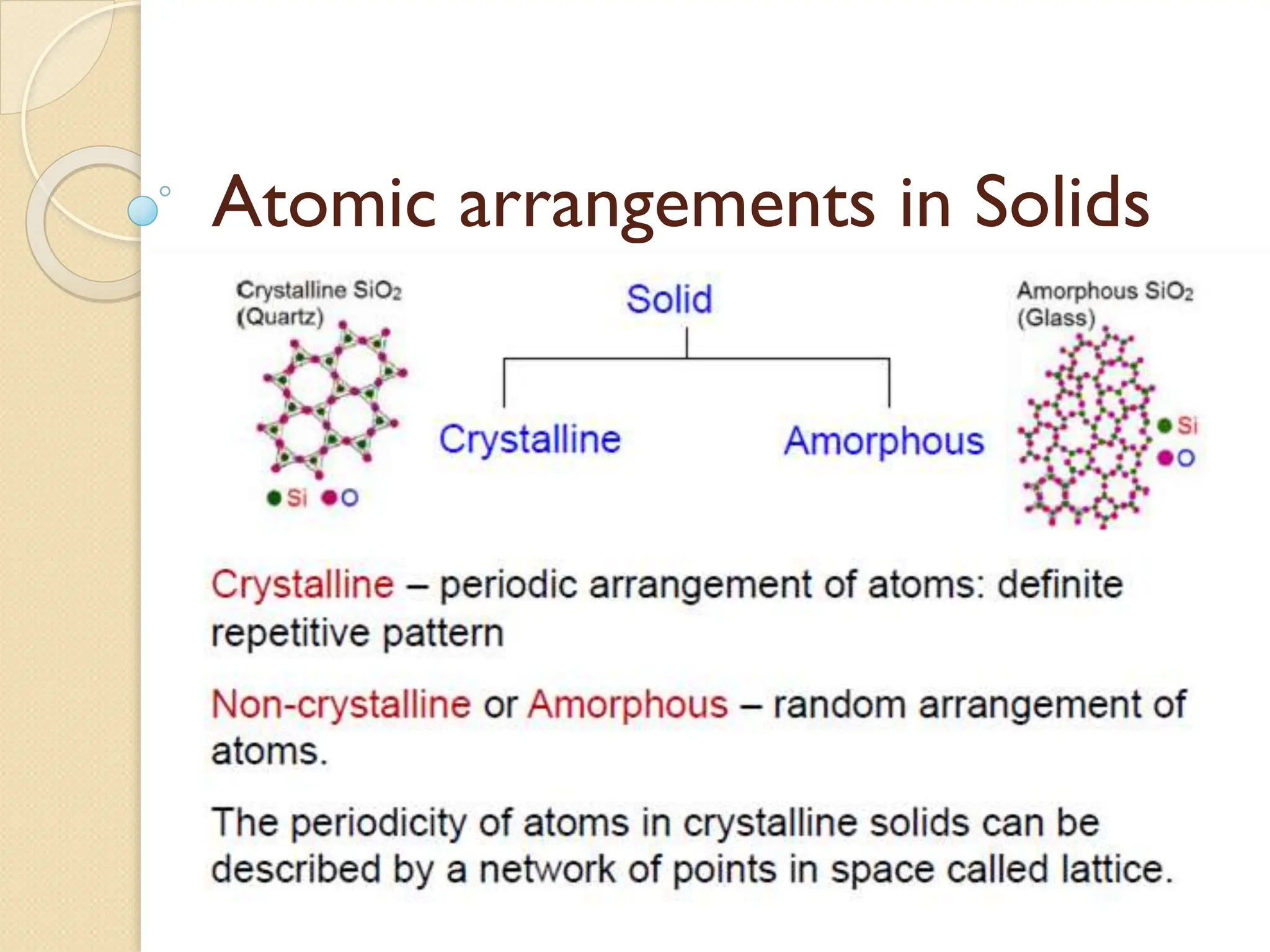
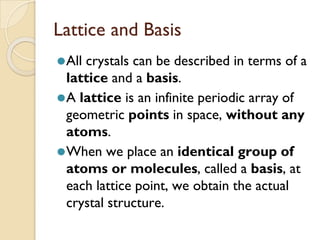
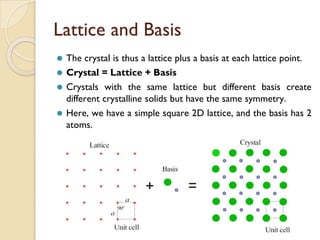
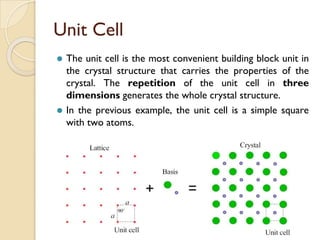
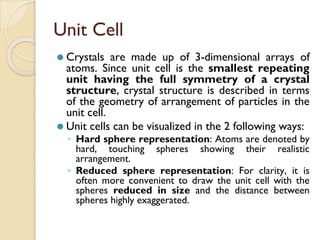
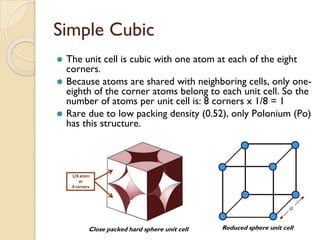
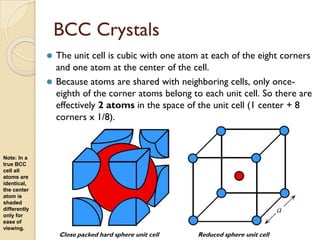
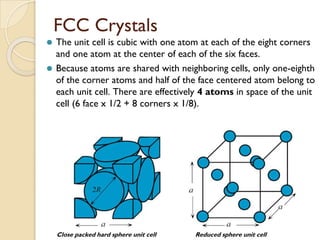
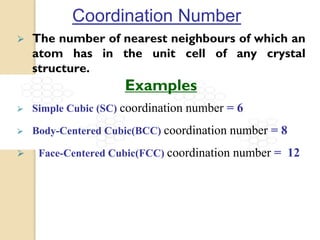
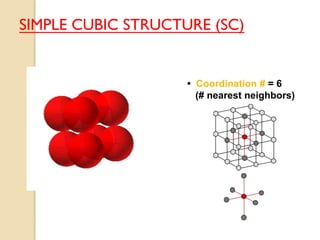
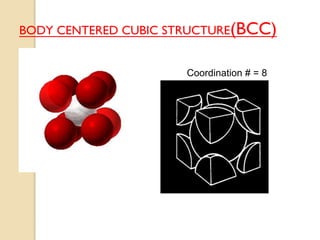
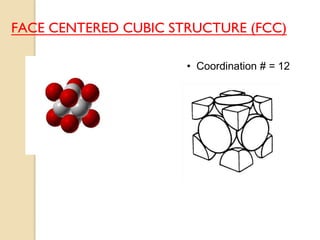
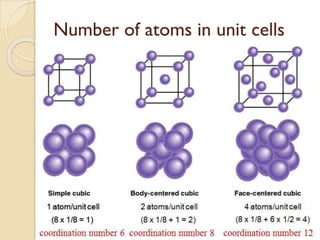
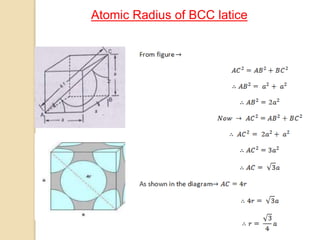
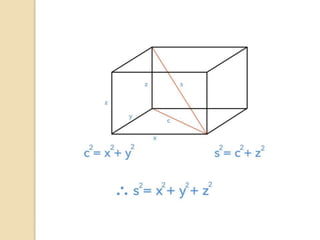
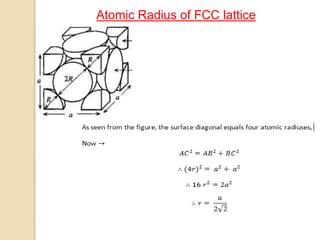
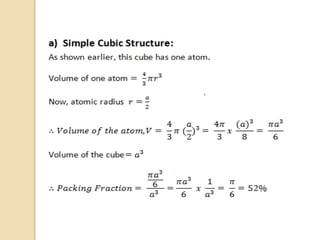
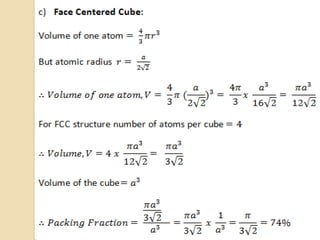
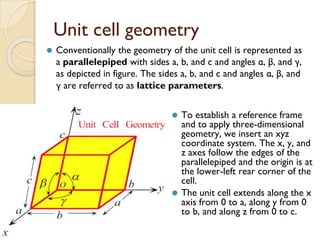
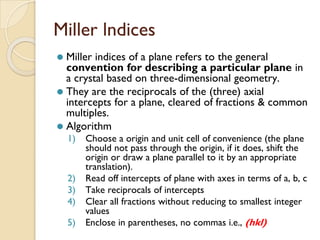
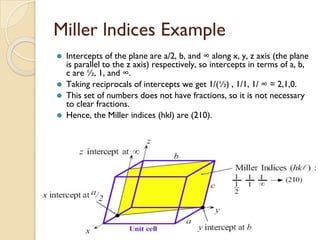
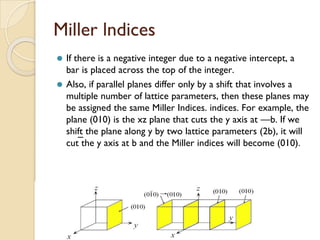
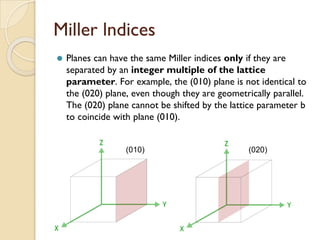
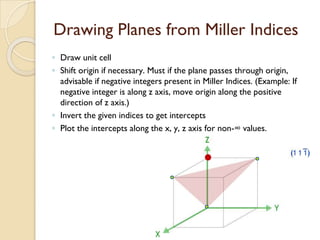
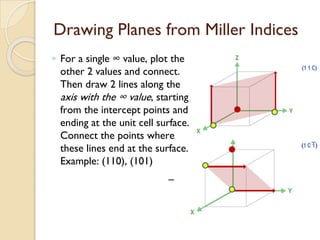
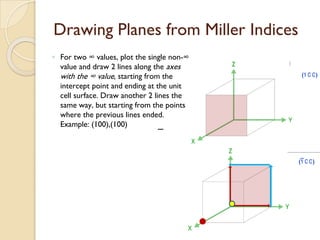
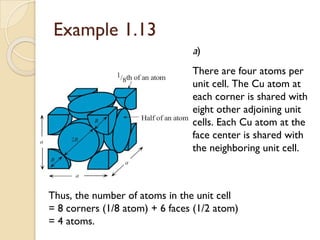
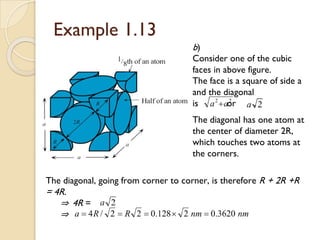
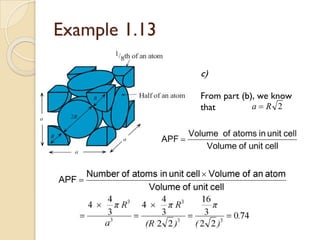
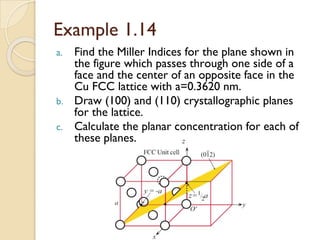
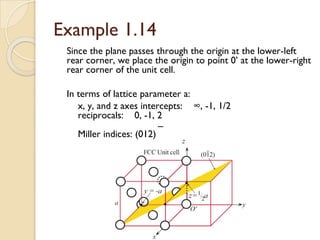
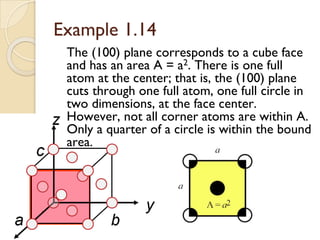
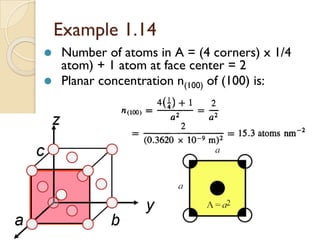
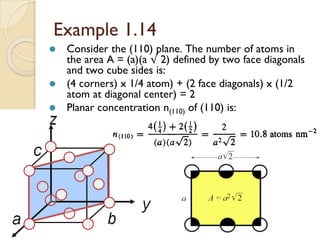
The document discusses the atomic arrangements in crystalline solids. It defines key concepts such as crystals, lattice, basis, unit cell, coordination number, Miller indices, and common crystal structures including simple cubic, body centered cubic, and face centered cubic. It provides examples of calculating the number of atoms in a unit cell, lattice parameter, atomic packing factor, atomic concentration, and drawing and identifying crystallographic planes from their Miller indices.