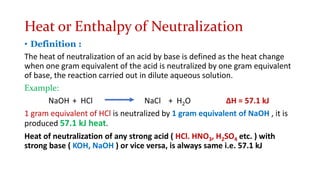
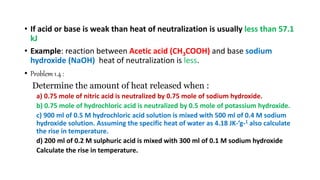
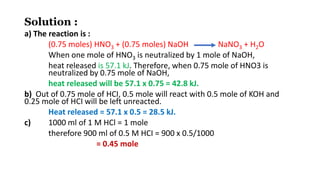
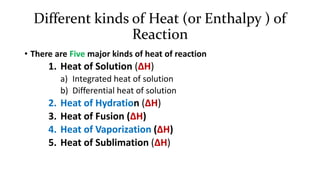
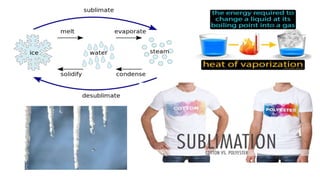
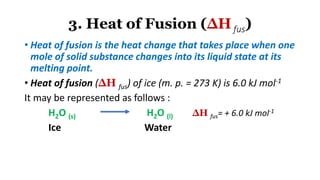
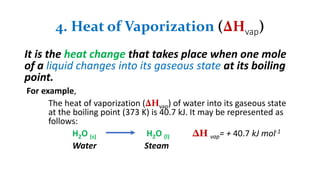
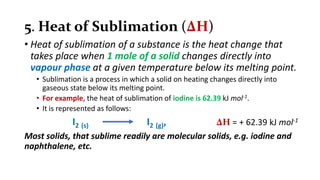
1. The document discusses heat (enthalpy) of various types of chemical reactions and processes, including heat of neutralization, heat of solution, heat of hydration, heat of fusion, heat of vaporization, and heat of sublimation.
2. It provides examples of calculating heat released during acid-base neutralization reactions and bond energies.
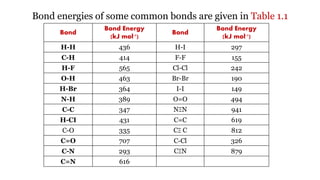
3. Bond energy is defined as the average amount of energy required to break bonds to form isolated gaseous atoms from molecules, or released when bonds are formed from atoms.






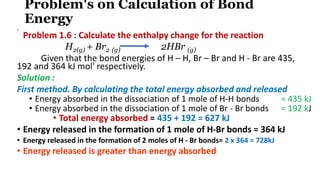
![Hence, net result is the release of energy.
Energy released = 728 kJ - 627 kJ = 101 kJ Thus, for the given
reaction,
∆H reaction = - 101 kJ
• Second method.
• Using the formula directly
• ∆H reaction = ∑ Bond energies of reactants - ∑ Bond energies of products
= [Bond energy (H-H) + Bond energy (Br - Br)] - [2 x Bond energy (H - Br)]
= 435 + 192 - (2 x 364)
= 627-728
∆H reaction = - 101 kJ.](https://image.slidesharecdn.com/chemicalenergetics04-211021051853/85/Chemical-energetics-04-17-320.jpg)
